Surface Morphology Control and Surface Enhanced Raman Scattering Effect of GO/Au/Ag Composite
-
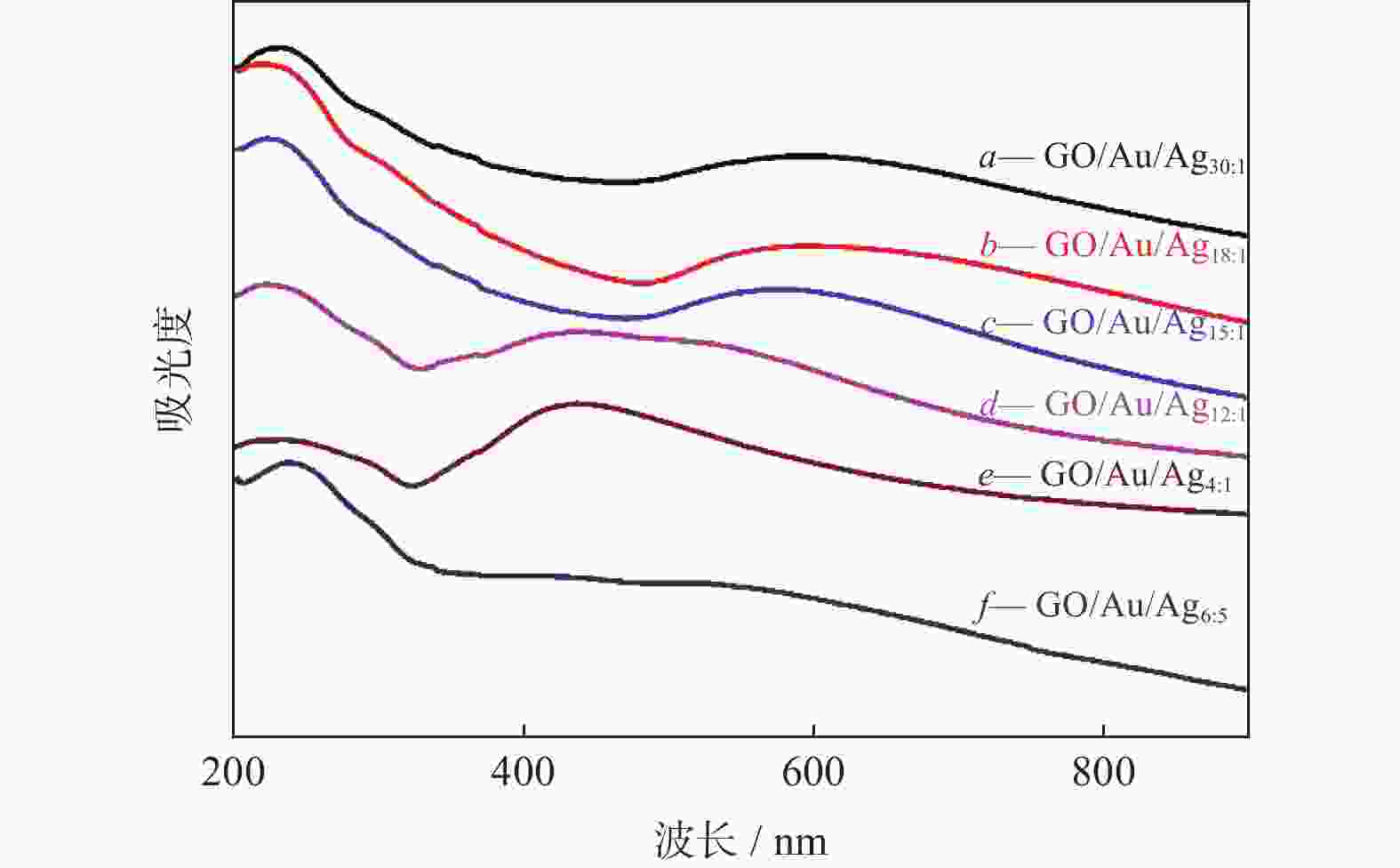
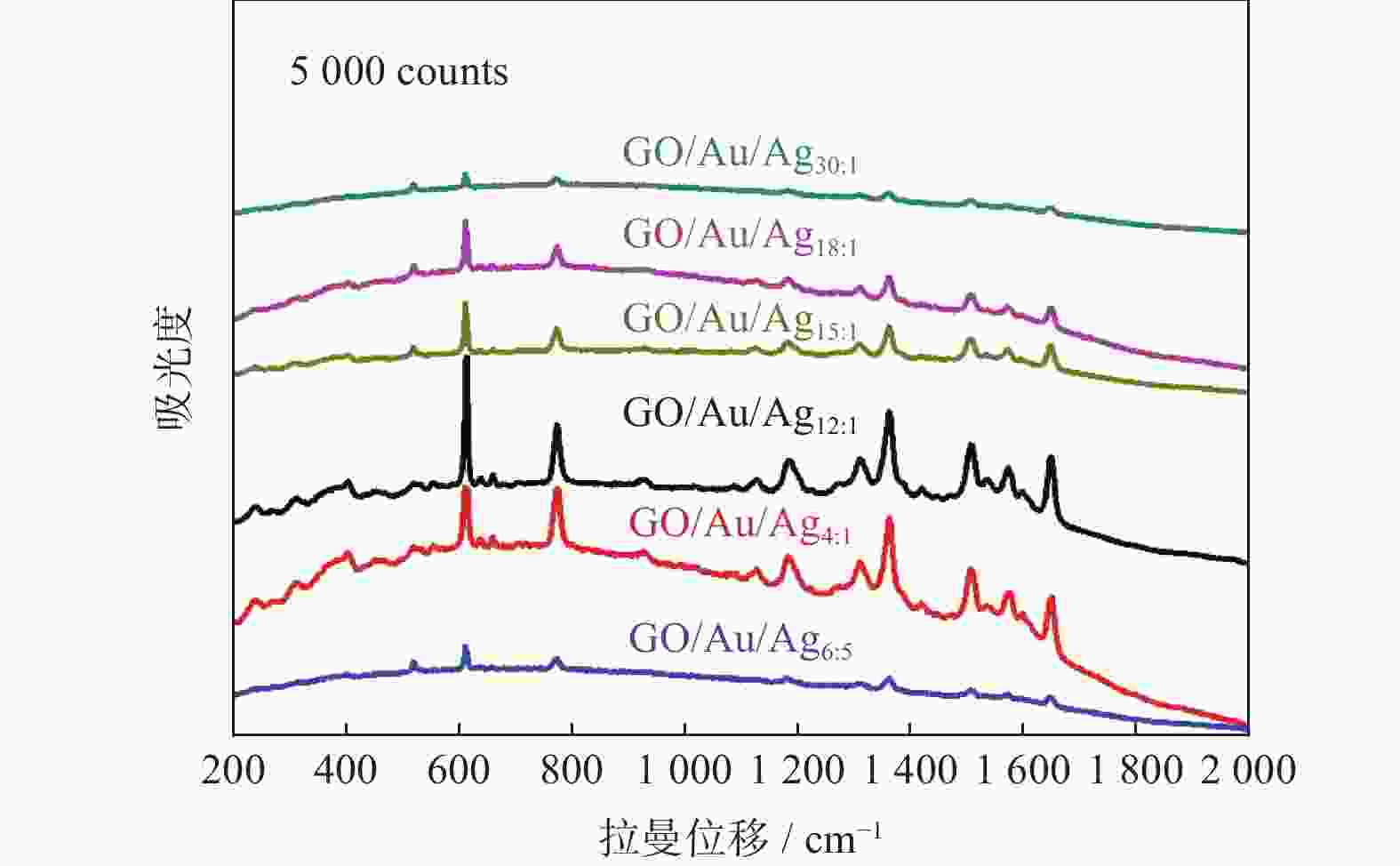
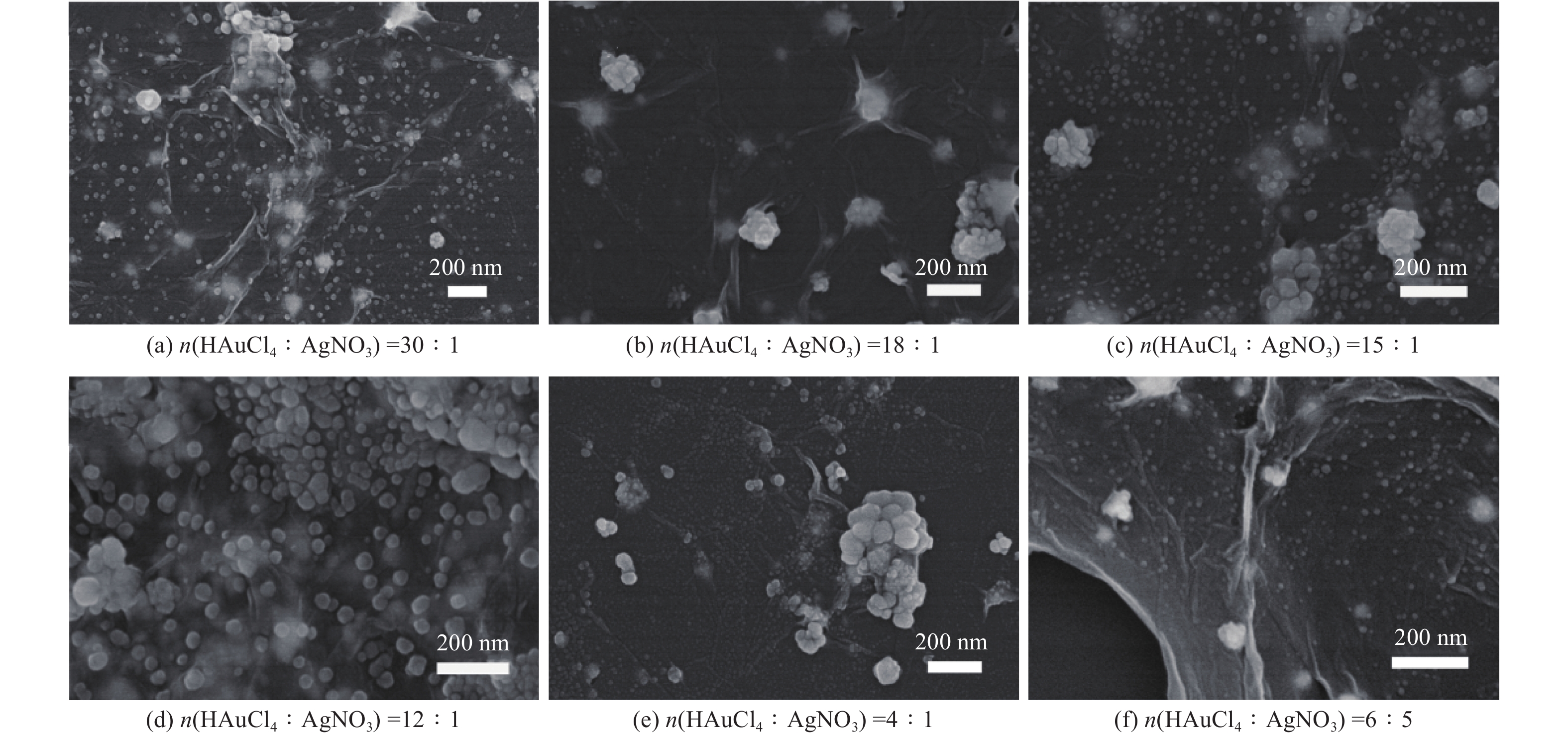
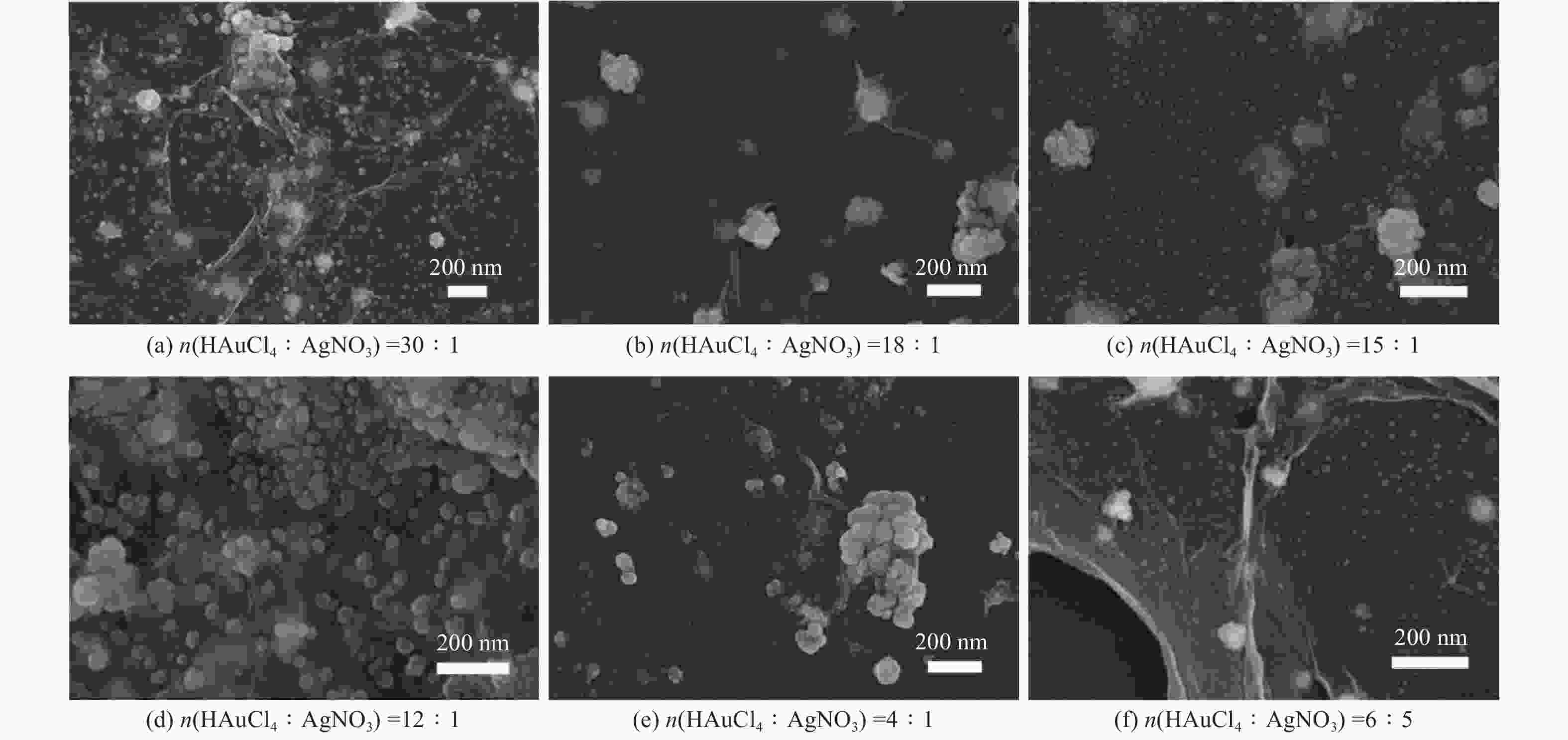
摘要: 表面增强拉曼散射光谱(SERS)因具有高灵敏及无损检测的特点,在化学检测领域受到广泛关注. 采用原位化学还原法,制备氧化石墨烯/金/银(GO/Au/Ag)复合材料,利用扫描电子显微镜(SEM)、X射线能谱仪(EDS)、紫光—可见分光光度计(UV)等手段对复合材料结构进行表征,并深入研究不同Au/Ag比例对纳米复合材料形貌和SERS的影响. 以罗丹明6G (R6G)为探针分子,研究纳米复合材料的形态对表面增强拉曼散射的影响. 研究结果表明GO/Au/Ag复合材料具有良好的SERS增强效果,且SERS信号强度与样品表面形貌以及Au、Ag含量(质量分数,全文同)有关. Au/Ag纳米颗粒表面粗糙度以及Au、Ag含量的提高可以显著增加GO/Au/Ag复合材料的SERS效果.
-
关键词:
- 氧化石墨烯/金/银(GO/Au/Ag) /
- 表面增强拉曼散射 /
- 表面形貌
Abstract: Surface enhanced Raman scattering (SERS) has attracted much attentions in the field of chemical detection because of its high sensitivity and nondestructive detection. Graphene oxide/Au/Ag (GO/Au/Ag) composites were prepared by in-situ chemical reduction method. Scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDS) and Ultraviolet-visible spctrophotometer (UV) were used to characterize the structure of the composites, and the effect of Au/Ag ratio on the morphology and SERS of composites were strudied. Rhodamine 6G (R6G) was used as the probe molecule to study the influence of composites morpfology on surface enhanced SERS. The result show that GO/Au/Ag composites have good SERS enhanced effect, and the SERS signal intensity is related to the surface morphology and the content (mass fraction) of Au and Ag. The increase of surface roughness of Au/Ag particles and the content of Au and Ag could significantly increased the SERS effect of GO/Au/Ag composites. -
表 1 GO/Au/Ag复合材料EDS图谱元素含量
Table 1. Element content of GO/Au/Ag composites based on EDS spectra
% 样品 C O Au Ag GO/Au/Ag30:1 52.25 28.59 13.58 5.59 GO/Au/Ag18:1 53.73 30.79 10.64 4.84 GO/Au/Ag15:1 55.26 21.13 11.65 11.97 GO/Au/Ag12:1 33.43 23.46 20.31 22.80 GO/Au/Ag4:1 36.94 26.19 16.69 20.19 GO/Au/Ag6:5 45.13 32.26 5.75 16.87 表 2 以GO/Au/Ag复合材料为基底时R6G拉曼位移及EF值
Table 2. Raman shift and EF values of R6G with substrates GO/Au/Ag composites as substrates
样品 拉曼位移 614 cm−1 EF 773 cm−1 EF 1186 cm−1 EF GO/Au/Ag30∶1 −4 0.70×104 −1 0.21×104 −4 0.21×104 GO/Au/Ag18∶1 −4 2.57×104 −1 0.85×104 −4 1.02×104 GO/Au/Ag15∶1 −4 2.60×104 −1 0.96×104 −4 1.47×104 GO/Au/Ag12∶1 −2 6.72×104 −1 2.60×104 −4 3.86×104 GO/Au/Ag4∶1 −4 3.06×104 −1 2.62×104 −4 4.20×104 GO/Au/Ag6∶5 −4 1.12×104 −1 0.44×104 −4 0.37×104 -
[1] FLEISCHMANN M, HENDRA P J, MCQUILLAN A J. Raman spectra of pyridine adsorbed at a silver electrode[J] . Chemical Physics Letters,1974,26(2):163 − 166. [2] LE RU E, BLACKIE E J, MEYER M, et al. Surface enhanced Raman scattering enhancement factors: a comprehensive study[J] . Journal of Physical Chemistry C,2007,111(37):13794 − 13803. [3] WANG K Q, SUN D W, PU H B, et al. Surface-enhanced Raman scattering of core-shell Au@Ag nanoparticles aggregates for rapid detection of difenoconazole in grapes[J] . Talanta,2019,191:449 − 456. [4] D’ANDREA C, FAZIO B, GUCCIARDI P G, et al. SERS enhancement and field confinement in nanosensors based on self-organized gold nanowires produced by ion-beam sputtering[J] . Journal of Physical Chemistry C,2014,118(118):8571 − 8580. [5] GUO P Z, SIKDAR D, HUANG X P, et al. Plasmonic core-shell nanoparticles for SERS detection of the pesticide thiram: size- and shape-dependent Raman enhancement[J] . Nanoscale,2015,7(7):2862 − 2868. [6] MILLO D, BONIFACIO A, MONCELLI M R, et al. Characterization of hybrid bilayer membranes on silver electrodes as biocompatible SERS substrates to study membrane–protein interactions[J] . Colloids and Surfaces B: Biointerfaces,2010,81(1):212 − 216. [7] BAI T T, SUN J F, CHE R C, et al. Controllable preparation of core–shell Au–Ag nanoshuttles with improved refractive index sensitivity and SERS activity[J] . ACS Applied Materials & Interfaces,2014,6(6):3331 − 3340. [8] MA P Y, LIANG F H, DIAO Q P, et al. Selective and sensitive SERS sensor for detection of Hg2+ in environmental water base on rhodamine-bonded and amino group functionalized SiO2-coated Au-Ag core-shell nanorods[J] . RSC Advances,2015,5(41):32168 − 32174. [9] HAN X X, CHEN L,KUHLMANN U, et al. Magnetic titanium dioxide nanocomposites for surface-enhanced resonance Raman spectroscopic determination and degradation of toxic anilines and phenols[J] . Angewandte Chemie International Edition,2014,53(53):2481 − 2484. [10] ZHANG J L,YANG H J,SHEN G X, et al. Reduction of graphene oxide via L-ascorbic acid[J] . Chemical Communications,2010,47(7):1112 − 150. [11] ALEKSANDRA W, KAMAT P V. Reduced graphene oxide and porphyrin. An interactive affair in 2-D[J] . ACS Nano,2010,4(11):6697 − 6706. [12] EMERY J D, WANG Q H, ZARROUATI M, et al. Structural analysis of PTCDA monolayers on epitaxial graphene with ultra-high vacuum scanning tunneling microscopy and high-resolution X-ray reflectivity[J] . Surface Science,2011,605(17-18):1685 − 1693. [13] XIE L M, LING X, FANG Y, et al. Graphene as a substrate to suppress fluorescence in resonance Raman spectroscopy[J] . Journal of the American Chemical Society,2009,131(29):9890 − 9891. [14] OTTO A. The ‘chemical’ (electronic) contribution to surface‐enhanced Raman scattering[J] . Journal of Raman Spectroscopy,2005,36(36):497 − 509. [15] ZHANG M J, LENG Y D, HUANG J, et al. Surface-enhanced Raman scattering of dipolar molecules by the graphene Fermi surface modulation with different dipole moments[J] . Applied Surface Science,2017,(425):654 − 662. [16] JUNG N, CROWTHER A C, KIM N, et al. Raman enhancement on graphene: adsorbed and intercalated molecular species[J] . ACS Nano,2010,4(11):7005 − 7013. [17] WANG L, ZHANG Y, YANG Y Q,et al. Strong dependence of surface enhanced Raman scattering on structure of graphene oxide film[J] . Materials,2018,11(7):1199. [18] ZHENG X L, PENG Y S, YANG Y, et al. Hydrothermal reduction of graphene oxide; effect on surface-enhanced Raman scattering[J] . Journal of Raman Spectroscopy,2017,48(48):97 − 103. [19] YANG H P, HU H L, NI Z H, et al. Comparison of surface-enhanced Raman scattering on graphene oxide, reduced graphene oxide and graphene surfaces[J] . Carbon,2013,(62):422 − 429. [20] YANG Y Q, WANG L, WANG Q S, et al. Synthesis of GO/Au/Ag nanocomposite with excellent surface enhanced Raman scattering effect[J] . Journal of Physics. Conference Series,2020,1622(1):012067. [21] HUMMERS W S, OFFEMAN R E. Preparation of graphitic oxide[J] . Journal of the American Chemical Society,1958,80(6):1339. [22] ROBINSON J T, TABAKMAN S M, LIANG Y Y, et al. Ultrasmall reduced graphene oxide with high near-infrared absorbance for photothermal therapy[J] . Journal of the American Chemical Society,2011,133(133):6825 − 6831. [23] 王玲, 张艳, 张婧, 等. Au@石墨烯量子点复合材料的制备及表面增强拉曼散射应用[J] . 新型炭材料,2019,34(6):606 − 610. [24] HIROYUKI W, NORIHIKO H, YASUSHI I, et al. DFT vibrational calculations of rhodamine 6G adsorbed on silver: analysis of tip-enhanced Raman spectroscopy[J] . Journal of Physical Chemistry B,2005(109):5012 − 5020. -





 下载:
下载: