Mechanism of a drug pair of Astragalus membranaceus and Lycium barbarum
-
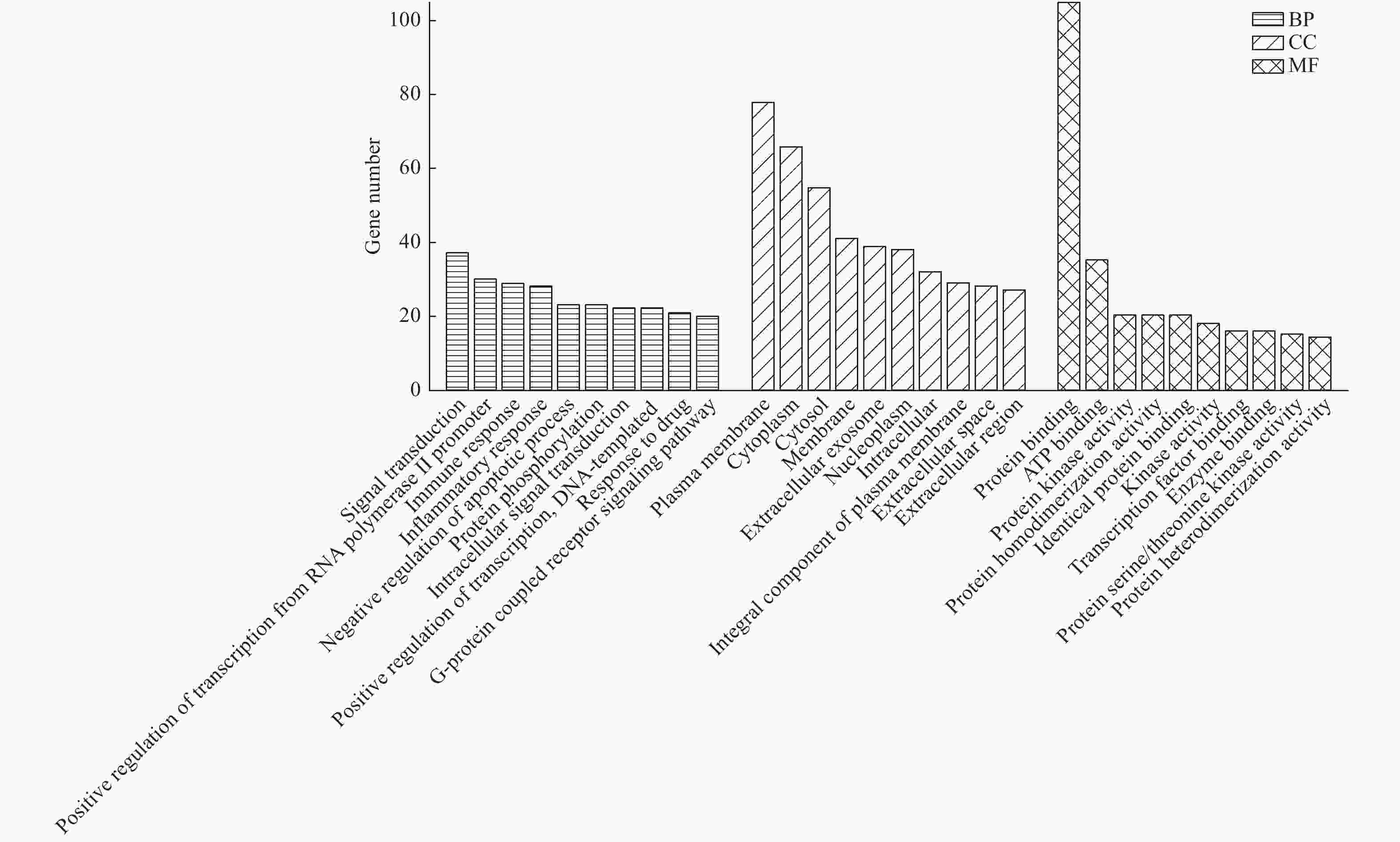
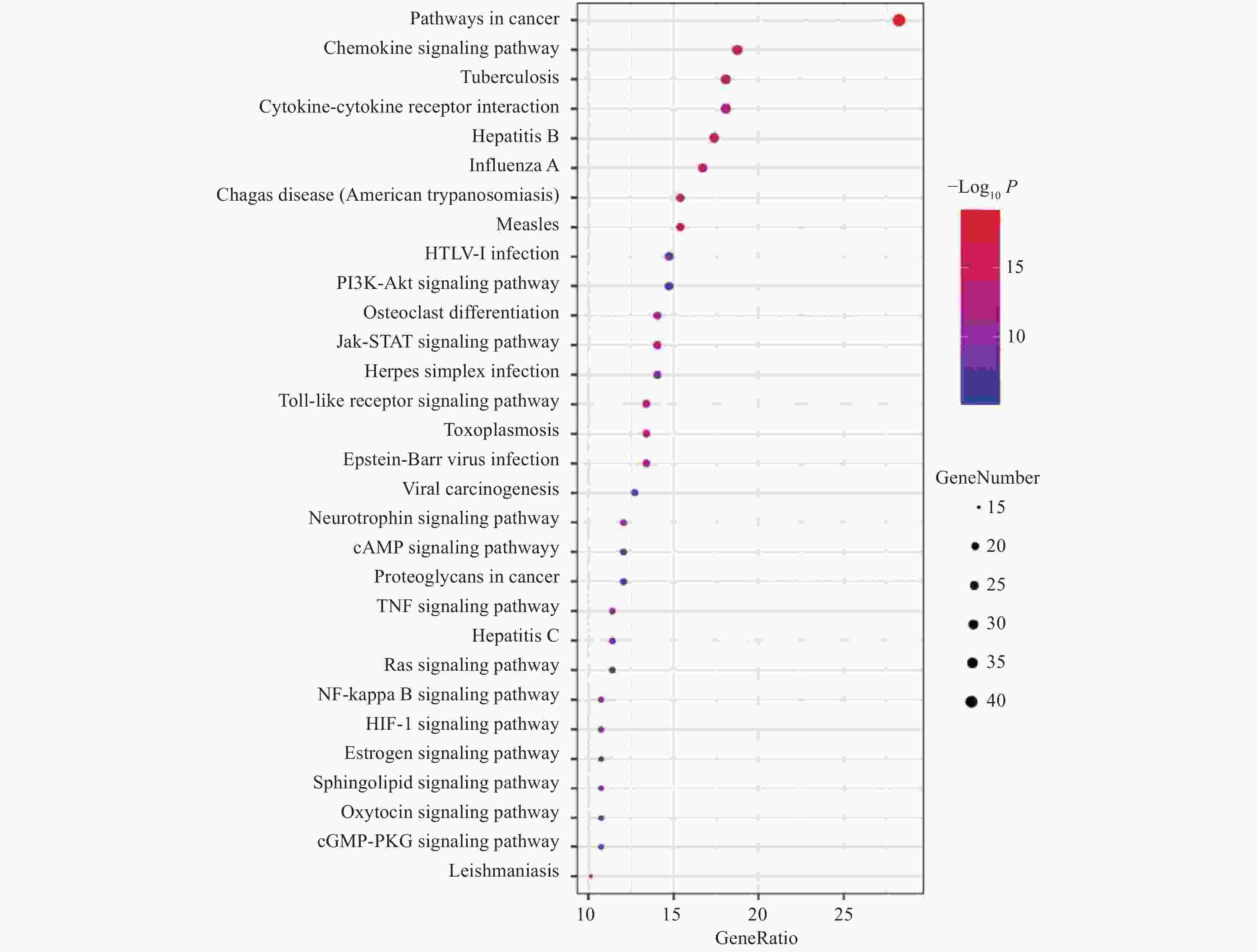
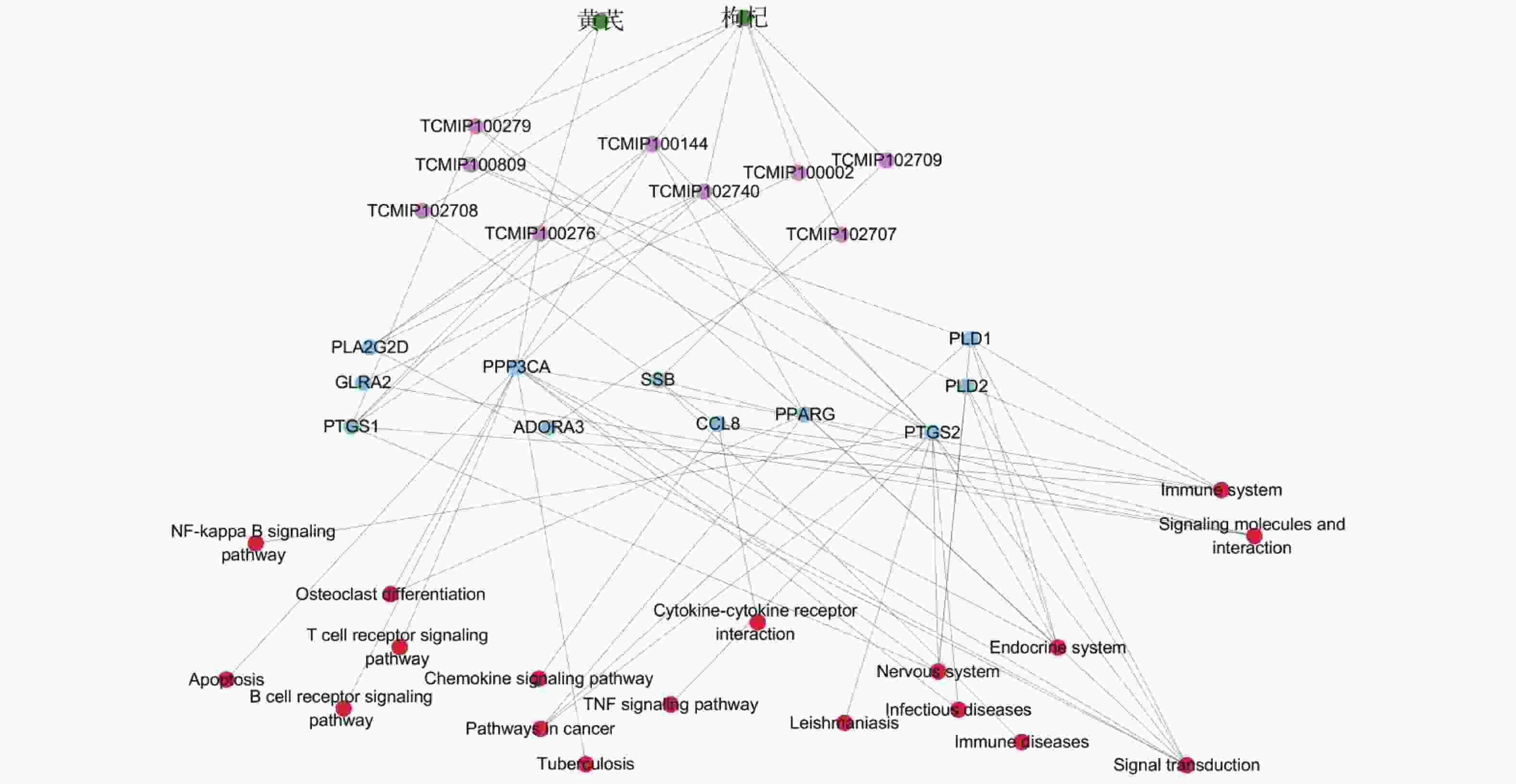
摘要: 黄芪−枸杞子药对治疗溃疡性结肠炎的临床疗效确切,然而作用机制不明. 采用网络药理学技术研究黄芪−枸杞子配伍抗溃疡性结肠炎的潜在作用机制. 利用整合药理学平台、String数据库、David 数据库等系统检索中药化学成分和候选靶标,通过分析药物和疾病蛋白靶标互作关系,筛选关键靶标并进行基因GO功能和KEGG通路富集分析,构建“中药−核心成分−关键靶标−主要通路”多维调控网络. 结果表明,黄芪−枸杞子可能通过作用于前列腺素 G/H 合成酶 2 (PTGS2) 等蛋白靶标,参与核转录因子(NF-κВ)、肿瘤坏死因子 (TNF) 等多个炎症和免疫相关信号通路,发挥抗溃疡性结肠炎作用. 研究结果为系统阐明黄芪−枸杞子药对治疗溃疡性结肠炎的作用机制提供参考.Abstract: The drug pair comprising of Astragalus membranaceus and Lycium barbarum (AL) has the clinical efficacy to prevent ulcerative colitis (UC). However, the mechanism of action is still arcane. The possible mechanism of this drug pair in the treatment of UC was investigated using a network pharmacology platform. The components and putative targets of AL were collected based on integrative pharmacology-based research platform of traditional Chinese medicine, String database, and David database. By analyzing the protein-protein interactive network containing the targets of AL and potential therapeutic targets of UC, GO and KEGG pathway enrichment analyses were performed, and a regulating network of herb-constituent-target-pathway was constructed. The results suggested that AL may play a beneficial role in UC through affecting the expression of targets associated with immune and inflammation such as PTGS2, and regulation of nuclear transcription factor-κВ signaling pathway, TNF signaling pathway, etc. The results of the study provide a reference for the systematic elucidation of the mechanism of AL in the treatment of UC.
-
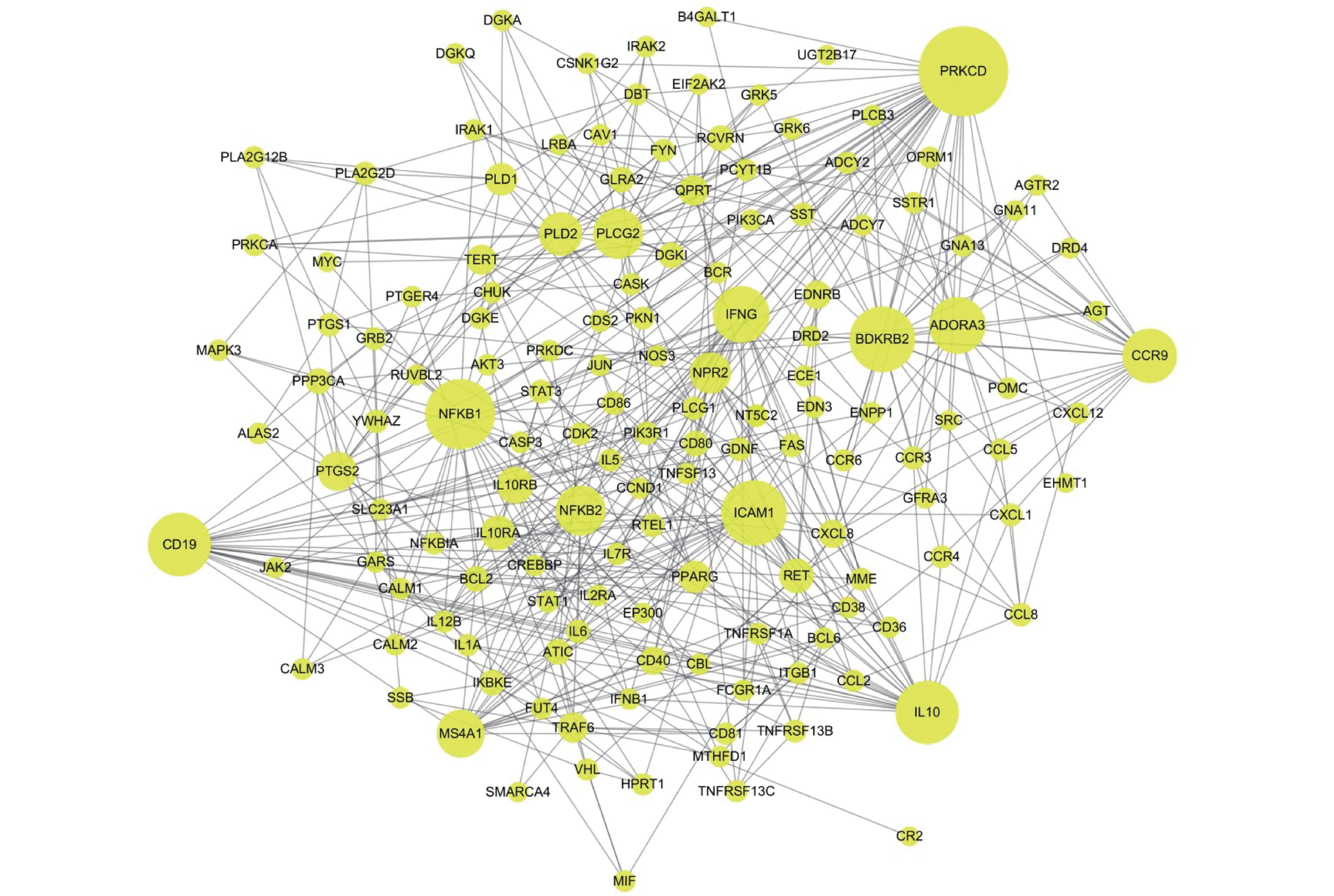
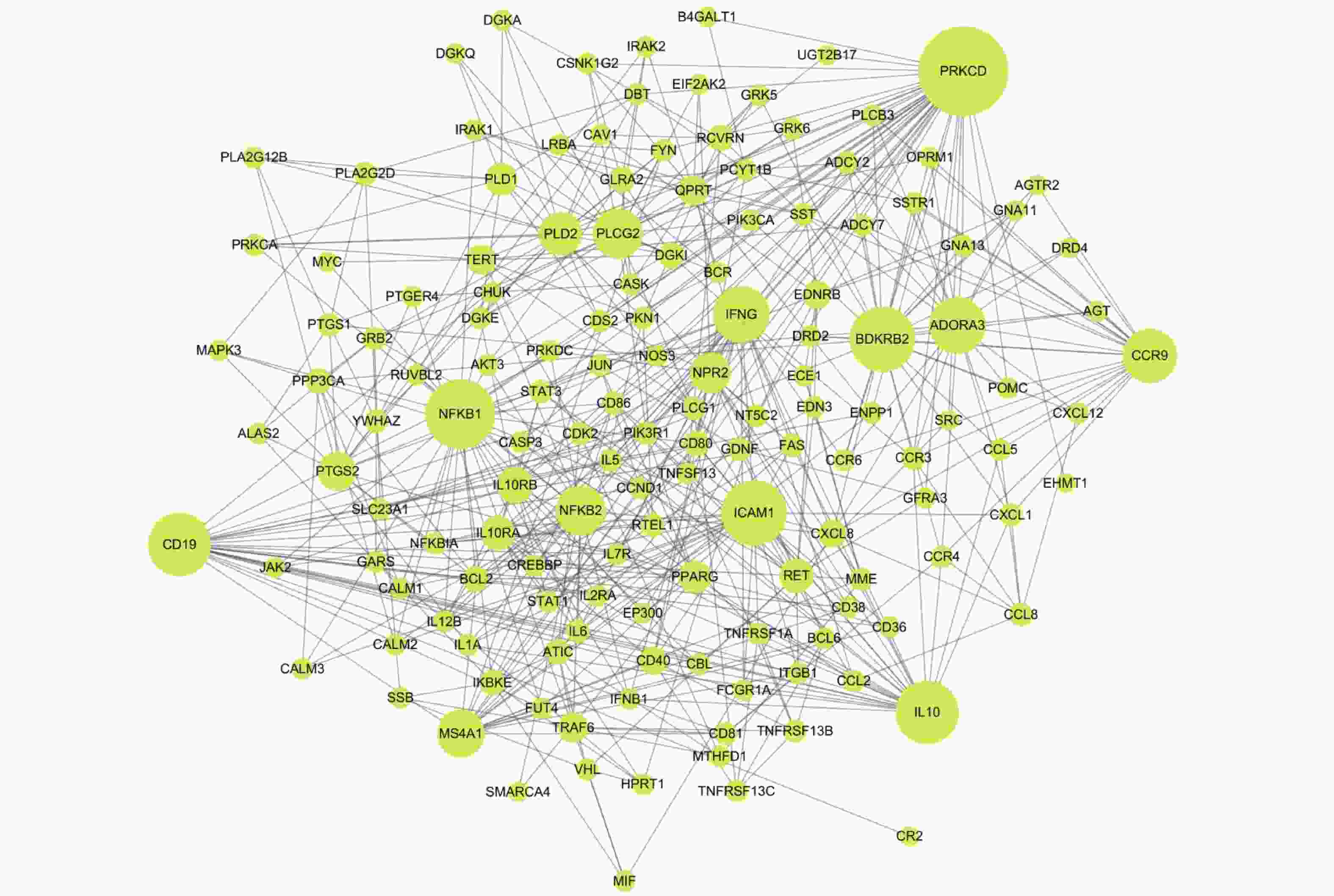
表 1 黄芪−枸杞子药对治疗溃疡性结肠炎关键靶标的网络拓扑特征参数
Table 1. Network topological parameters of key targets of Astragalus membranaceus and Lycium barbarum for UC
序号 关键网络靶标 连接度 紧密度 介度 序号 关键网络靶标 连接度 紧密度 介度 1 PRKCD 37 0.477 16.490 13 PLD2 16 0.416 3.337 2 BDKRB2 26 0.397 2.986 14 NPR2 15 0.405 2.431 3 NFKB1 26 0.424 6.872 15 PTGS2 14 0.377 2.389 4 IL10 25 0.422 6.146 16 IL10RB 13 0.395 1.694 5 CD19 25 0.419 3.396 17 RET 12 0.378 3.653 6 ICAM1 24 0.405 3.299 18 IL10RA 12 0.345 0.062 7 ADORA3 22 0.376 0 19 PLD1 11 0.334 0.142 8 IFNG 22 0.414 1.198 20 PPARG 11 0.367 1.135 9 CCR9 21 0.375 6.438 21 QPRT 10 0.343 0.153 10 PLCG2 19 0.383 6.306 22 TRAF6 10 0.351 2.340 11 MS4A1 18 0.396 2.087 23 TERT 10 0.390 3.559 12 NFKB2 17 0.393 4.493 -
[1] ORDÁS I, ECKMANN L, TALAMINI M, et al. Ulcerative colitis[J] . Lancet,2012,380(9853):1606 − 1619. doi: 10.1016/S0140-6736(12)60150-0 [2] THOMAS T, ABRAMS K A, ROBINSON R J, et al. Meta-analysis: Cancer risk of low-grade dysplasia in chronic ulcerative colitis[J] . Alimentry Pharmacology and Therapeutics,2007,25(6):657 − 668. doi: 10.1111/j.1365-2036.2007.03241.x [3] PASTORELLI L, PIZARRO T T, COMINELLI F, et al. Emerging drugs for the treatment of ulcerative colitis[J] . Expert Opinion on Emerging Drugs,2009,14(3):505 − 521. doi: 10.1517/14728210903146882 [4] JIANG X L, CUI H F. An analysis of 10218 ulcerative colitis cases in China[J] . World Journal of Gastroenterology,2002,8(1):158 − 161. doi: 10.3748/wjg.v8.i1.158 [5] ZHAO L J, WU H B, ZHAO A H, et al. The in vivo and in vitro study of polysaccharides from a two-herb formula on ulcerative colitis and potential mechanism of action[J] . Journal of Ethnopharmacology: An Interdisciplinary Journal Devoted to Bioscientific Research on Indigenous Drugs,2014,153(1):151 − 159. doi: 10.1016/j.jep.2014.02.008 [6] HOPKINS A L. Network pharmacology[J] . Nature Biotechnology,2007,25(10):1110 − 1111. doi: 10.1038/nbt1007-1110 [7] WANG X, WANG Z Y, ZHENG J H, et al. TCM network pharmacology: A new trend towards combining computational, experimental and clinical approaches[J] . Chinese Journal of Natural Medicines,2021,19(1):1 − 11. [8] XU H Y, ZHANG Y Q, LIU Z M, et al. ETCM: An encyclopaedia of traditional Chinese medicine[J] . Nucleic Acids Research,2019,47(D1):D976 − D982. doi: 10.1093/nar/gky987 [9] 章轶立, 唐彬, 姜俊杰, 等. 整合药理学视角下的骨碎补治疗骨质疏松症作用机制研究[J] . 中国中药杂志,2018,43(20):4125 − 4131. [10] 黄晓燕, 邹孟龙, 陈雅璐, 等. 基于网络药理学和分子对接分析黄芪治疗溃疡性结肠炎的作用机制[J] . 中药新药与临床药理,2021,32(6):815 − 824. [11] LV J, ZHANG Y H, TIAN Z Q, et al. Astragalus polysaccharides protect against dextran sulfate sodium-induced colitis by inhibiting NF-κВ activation[J] . International Journal of Biological Macromolecules,2017,98(1):723 − 729. [12] KANG Y F, XUE Y S, DU M, et al. Preventive effects of Goji berry on dextran-sulfate-sodium-induced colitis in mice[J] . The Journal of Nutritional Biochemistry,2017,40(1):70 − 76. [13] SHARMA A, TIRPUDE N V, KULURKAR P M, et al. Berberis lycium fruit extract attenuates oxi-inflammatory stress and promotes mucosal healing by mitigating NF-κB/c-Jun/MAPKs signalling and augmenting splenic Treg proliferation in a murine model of dextran sulphate sodium-induced ulcerative colitis[J] . European Journal of Nutrition,2020,59(6):2663 − 2681. doi: 10.1007/s00394-019-02114-1 [14] ZONG S, YANG L, PARK H J, et al. Dietary intake of Lycium ruthenicum Murray ethanol extract inhibits colonic inflammation in dextran sulfate sodium-induced murine experimental colitis[J] . Food & Function,2020,11(4):2924 − 2937. doi: 10.1039/D0FO00172D -





 下载:
下载: