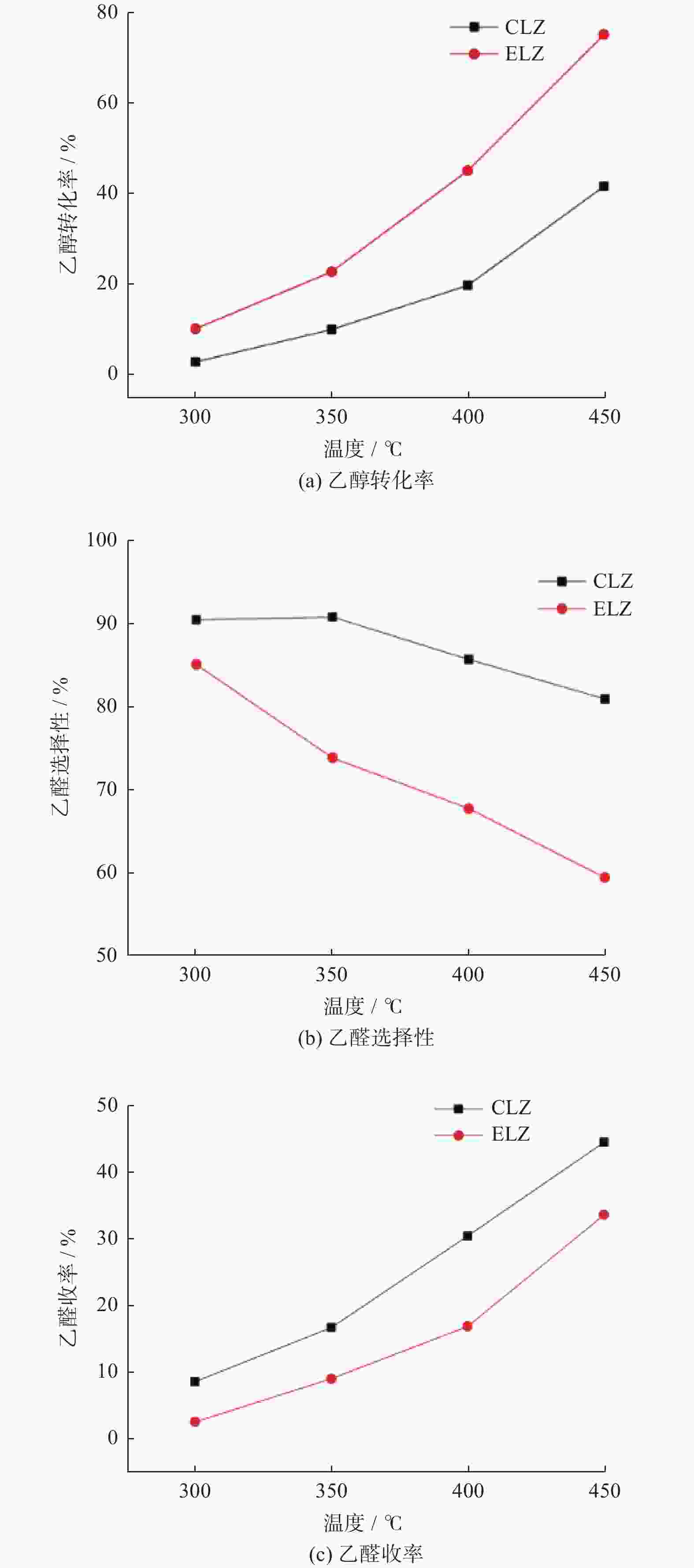
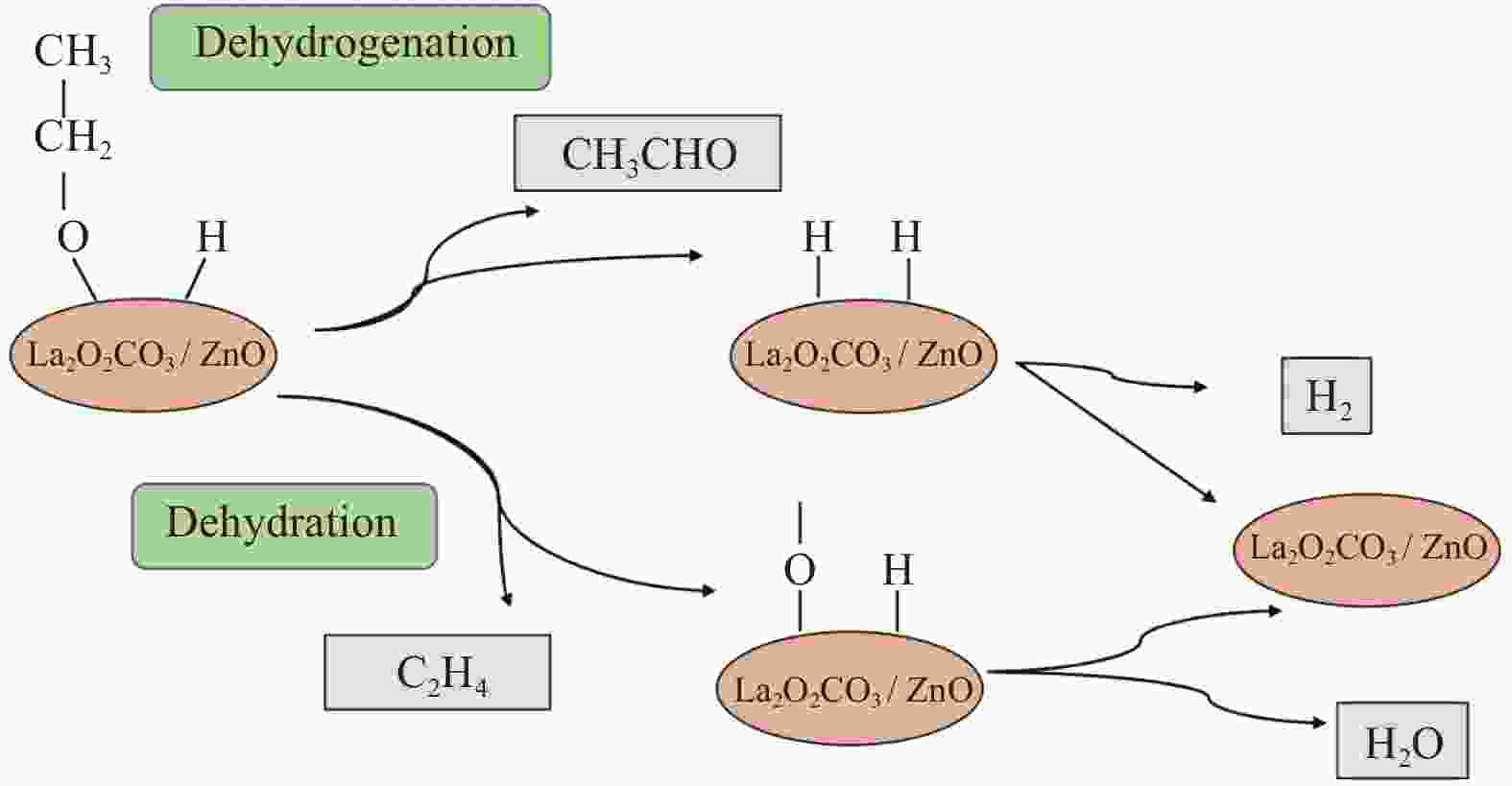
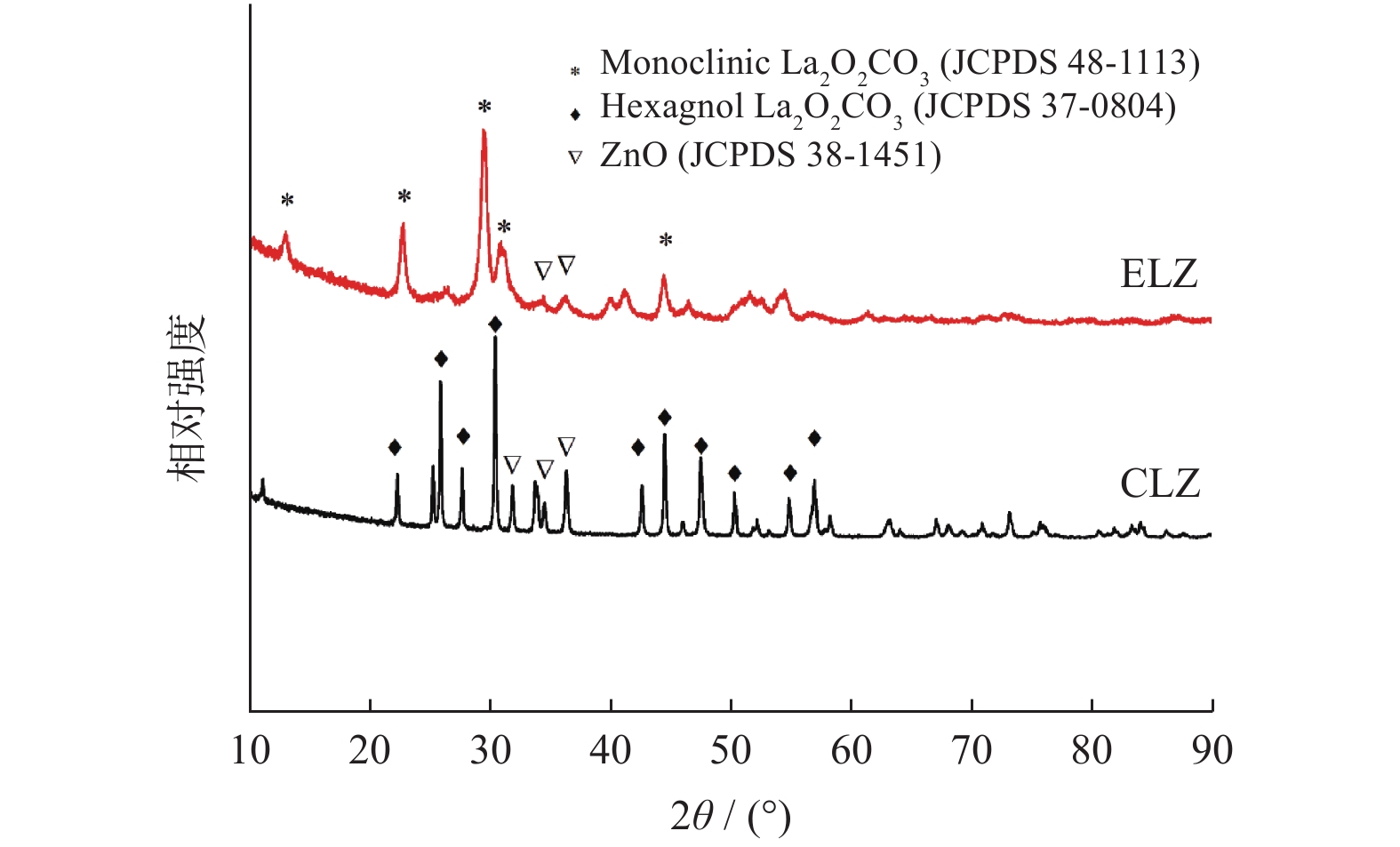
|
[1]
|
GONZALEZ-GARCIA S, LOU L, MOREIRA M T, et al. Life cycle assessment of hemp hurds use in second generation ethanol production[J] . Biomass & Bioenergy,2012,36:268 − 279.
|
|
[2]
|
JOHN R P, ANISHA G S, NAMPOOTHIRI K M, et al. Micro and macroalgal biomass: A renewable source for bioethanol[J] . Bioresource Technology,2011,102(1):186 − 193. doi: 10.1016/j.biortech.2010.06.139
|
|
[3]
|
GHASEMZADEH K, JALILNEJAD E, BASILE A. 3-Production of bioalcohol and biomethane[J] . Bioenergy Systems for the Future,2017:61 − 86.
|
|
[4]
|
PAMPARARO G, GARBARINO G, RIANI P, et al. A study of ethanol dehydrogenation to acetaldehyde over supported copper catalysts: catalytic activity, deactivation and regeneration[J] . Applied Catalysis A: General,2020, 602:117710.
|
|
[5]
|
MOSTROU S, SIPŐCZ T, NAGL A, et al. Catalytic oxidation of aqueous bioethanol: An efficient upgrade from batch to flow[J] . Reaction Chemistry & Engineering,2018,3(5):781 − 789.
|
|
[6]
|
HAGEMEYER H J. Kirk-Othmer Encyclopedia of Chemical Technology[M]. 5th Edition. Hoboken: John Wiley & Sons, 2002: 564 − 575.
|
|
[7]
|
GUAN Y, HENSEN E J M. Ethanol dehydrogenation by gold catalysts: The effect of the gold particle size and the presence of oxygen[J] . Applied Catalysis A: General,2009,361(1/2):49 − 56.
|
|
[8]
|
CHANG F W, YANG H C, ROSELIN L S, et al. Ethanol dehydrogenation over copper catalysts on rice husk ash prepared by ion exchange[J] . Applied Catalysis A General,2006,304:30 − 39. doi: 10.1016/j.apcata.2006.02.017
|
|
[9]
|
LI S X, MEN Y, WANG J G, et al. Morphological control of inverted MgO-SiO2 composite catalysts for efficient conversion of ethanol to 1, 3-butadiene[J] . Applied Catalysis A General,2019,577:1 − 9. doi: 10.1016/j.apcata.2019.03.007
|
|
[10]
|
WANG X F, MEN Y, WANG J G, et al. The influence of zinc loadings on the selectivity control of bio-ethanol transformation over MgO-SiO2 catalysts[J]. Applied Catalysis A: General, 2020, 598: 117565. DOI: 10.1016/j.apcata.2020.117565
|
|
[11]
|
SATO A G, VOLANTI D P, FREITAS I, et al. Site-selective ethanol conversion over supported copper catalysts[J] . Catalysis Communications,2012,26:122 − 126. doi: 10.1016/j.catcom.2012.05.008
|
|
[12]
|
FREITAS I C, DAMYANOVA S, OLIVEIRA D C, et al. Effect of Cu content on the surface and catalytic properties of Cu/ZrO2 catalyst for ethanol dehydrogenation[J] . Journal of Molecular Catalysis A: Chemical,2014,381(1):26 − 37.
|
|
[13]
|
DEWILDE J F, CZOPINSKI C J, BHAN A. Ethanol dehydration and dehydrogenation on γ-Al2O3: Mechanism of acetaldehyde formation[J] . ACS Catalysis,2014,4(12):4425 − 4433. doi: 10.1021/cs501239x
|
|
[14]
|
SKINNER M J, MICHOR E L, FAN W, et al. Ethanol dehydration to ethylene in a stratified autothermal millisecond reactor[J] . ChemSusChem,2011,4(8):1151 − 1156. doi: 10.1002/cssc.201100026
|
|
[15]
|
AUTTHANIT C, JONGSOMJIT B. Production of Ethylene through Ethanol Dehydration on SBA-15 Catalysts Synthesized by Sol-gel and One-step Hydrothermal Methods[J] . Journal of oleo science,2018,67(2):235 − 243. doi: 10.5650/jos.ess17167
|
|
[16]
|
LA-SALVIA N, LOVON-QUINTANA JJ, VALENCA GP. Vapor-Phase Catalytic Conversion of Ethanol into 1, 3-Butadiene on Cr-Ba/MCM-41 Catalysts[J] . Brazilian Journal of Chemical Engineering,2015,32(2):489 − 500. doi: 10.1590/0104-6632.20150322s00003039
|
|
[17]
|
DING D G, LU W B, XIONG Y, et al. Facile synthesis of La2O2CO3 nanoparticle films and Its CO2 sensing properties and mechanisms[J] . Applied Surface Science,2017,426 (31):725 − 733. doi: 10.1016/j.apsusc.2017.07.126
|
|
[18]
|
PARK C Y, NGUYEN-PHU H, SHIN E W. Glycerol carbonation with CO2 and La2O2CO3/ZnO catalysts prepared by two different methods: Preferred reaction route depending on crystalline structure[J] . Molecular Catalysis,2017,435:99 − 109. doi: 10.1016/j.mcat.2017.03.025
|
|
[19]
|
LI H G, GAO D Z, GAO P, et al. The synthesis of glycerol carbonate from glycerol and CO2 over La2O2CO3-ZnO catalysts[J] . Catalysis Science & Technology,2013,3:2801 − 2814.
|
|
[20]
|
LI H, Xi J, Lei L, et al. Synthesis of glycerol carbonate by direct carbonylation of glycerol with CO2 over solid catalysts derived from Zn/Al/La and Zn/Al/La/M (M = Li, Mg and Zr) hydrotalcites[J] . Catalysis Science & Technology,2015,5:989 − 996.
|
|
[21]
|
JIN L, ZHANG Y, DOMBROWSKI J P, et al. ZnO/La2O2CO3 layered composite: A new heterogeneous catalyst for the efficient ultra-fast microwave biofuel production[J] . Applied Catalysis B Environmental,2011,103(1/2):200 − 205. doi: 10.1016/j.apcatb.2011.01.027
|
|
[22]
|
ZHANG G, ZHAO Z, LIU J, et al. Macroporous perovskite-type complex oxide catalysts of La1– xKxCo1– yFeyO3 for diesel soot combustion[J] . Journal of Rare Earths,2009,27(6):955 − 960. doi: 10.1016/S1002-0721(08)60369-5
|
|
[23]
|
YU H Y, MEN Y, SHIN E W. Structural properties of disordered macroporous La2O2CO3/ZnO materials prepared by a solution combustion method[J] . Korean Journal of Chemical Engineering,2019,36(4):522 − 528. doi: 10.1007/s11814-019-0239-5
|
|
[24]
|
IRUSTA S, CORNAGLIA L M, LOM BA RDO E A. Effects of rhodium and platinum on the reactivity of lanthanum phases[J] . Materials Chemistry & Physics,2004,86(2/3):440 − 447.
|
|
[25]
|
TURCOTTE R P, SAWYER J O, EYRING L R. On the rare earth dioxymonocarbonates and their decomposition[J] . Inorganic Chemistry,1969,8(2):238 − 246. doi: 10.1021/ic50072a012
|
|
[26]
|
LEVAN T, CHE M, TATIBOUET J M, et al. Infrared study of the formation and stability of La2O2CO3 during the oxidative coupling of methane on La2O3[J] . Journal of Catalysis,1993,142(1):18 − 26. doi: 10.1006/jcat.1993.1185
|
|
[27]
|
NI J, CHEN L, LIN J, et al. High performance of Mg-La mixed oxides supported Ni catalysts for dry reforming of methane: The effect of crystal structure[J] . International Journal of Hydrogen Energy,2013,38(31):13631 − 13642. doi: 10.1016/j.ijhydene.2013.08.041
|
|
[28]
|
PAKHARE D, SCHWARTZ V, ABDELSAYED V, et al. Kinetic and mechanistic study of dry (CO2) reforming of methane over ROOH-substituted La2Zr2O7 pyrochlores[J] . Journal of Catalysis,2014,316:78 − 92. doi: 10.1016/j.jcat.2014.04.023
|
|
[29]
|
KVISLE S, AGUERO A, SNEEDEN R. Transformation of ethanol into 1, 3-butadiene over magnesium oxide/silica catalysts[J] . Applied Catalysis,1988,43(1):117 − 131. doi: 10.1016/S0166-9834(00)80905-7
|





 下载:
下载: