GC-MS analysis, antimicrobial and antioxidant activities of volatile constituents from Isatidis Radix (Banlangen)
-
摘要:
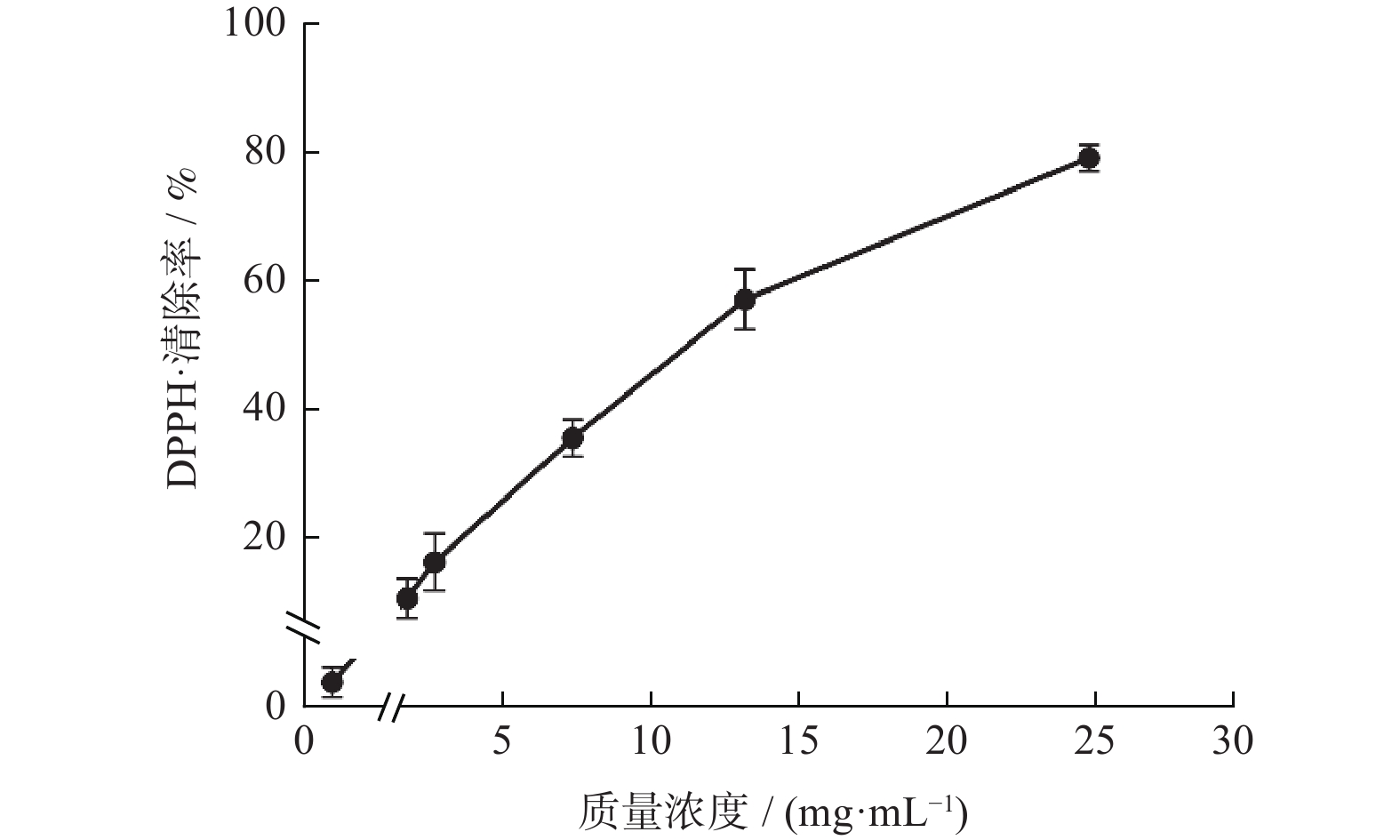
通过气相色谱–质谱 (GC-MS) 对板蓝根挥发性成分的化学组成进行分析,并通过 Kirby-Bauer 药敏纸片法、普鲁士蓝法、铁还原抗氧化能力 (FRAP) 和 1,1-二苯基-2-三硝基苯肼自由基 (DPPH·) 清除法研究其体外抗菌和抗氧化活性. 结果表明,板蓝根挥发性成分的质量分数较低 (0.038%),通过NIST数据库分析从中鉴定出60种化学成分,主要包括异硫氰酸酯、芳香烃类、羧酸类等. 板蓝根挥发性成分对大肠杆菌、金黄色葡萄球菌有一定抑菌作用,平均抑菌圈直径分别为8.1和6.5 mm;板蓝根挥发性成分具有较好的还原能力,对DPPH自由基清除效果明显, IC50值为7.169 mg/mL.
Abstract:The aim of this study was to determine the chemical constituents of volatile constituents from Isatidis Radix, and evaluate the antimicrobial and antioxidant activities using in vitro assays, including disk diffusion test, prussian blue test, ferric reducing antioxidant power and 1, 1-diphenyl-2-trinitrophenylhydrazine free radical (DPPH·) scavenging activity. Results showed that the volatile constituents of Isatidis Radix had low content (0.038%) and comprised of isothiocyanates, aromatic hydrocarbons, carboxylic acids, etc., which were identified by NIST Library. The volatile constituents of Isatidis Radix had antibacterial effects on Escherichia coli and Staphylococcus aureus, and the average antibacterial zone diameters were 8.1 mm and 6.5 mm, respectively. Also, it had good reducing ability, and obvious scavenging effect on DPPH·, with IC50 value of 7.169 mg/mL.
-
Key words:
- Isatidis Radix /
- volatile constituents /
- GC-MS analysis /
- antimicrobial activity /
- antioxidant activity
-
表 1 板蓝根挥发性成分的GC-MS分析结果
Table 1. GC-MS analysis result of volatile constituents from Isatidis Radix
名称 保留时间 /min 相似度 相对含量/% 名称 保留时间 /min 相似度 相对含量/% 羟基乙酸 4.75 737 trace 2,3−二氢−1,6−二甲基−1H−茚 14.83 758 0.04 3−丁烯基异硫氰酸酯 6.84 899 45.69 甲基苯甲酸乙酯 15.07 873 0.33 1,2,3−三甲基苯 7.18 932 0.04 1−亚乙基−1H−茚 15.30 869 0.62 3−甲基−1−乙基苯 7.85 930 1.35 庚酸 17.05 715 0.11 1−甲基4−异丙基−环己烯 8.05 816 0.02 联二苯 17.49 812 0.03 二氢化茚 8.21 895 0.48 1,2−二甲基萘 18.51 780 0.04 1−甲基−4丙基苯 8.59 885 0.28 α−法尼烯 18.55 755 0.06 4−甲基苯乙醛 8.73 779 1.24 2−羟基−4−甲氧苯基乙酮 19.02 834 0.10 1,2−二乙苯 8.75 745 1.50 2−甲氧基−4−丙烯酚 19.18 737 0.05 1−异丁基甲苯 8.97 728 0.01 异硫氰酸−2−苯基乙酯 19.59 892 1.23 4−丙基甲苯 8.97 886 0.97 1,5−二甲基−4−乙烯基−4−甲苯 20.02 716 0.09 1,2−二甲基−2−乙苯 9.31 807 0.01 香橙烯 20.60 769 0.08 2−异丙基甲苯 9.32 926 0.02 二去氢菖蒲烯 20.77 762 0.08 3−羟基−3,7−二甲基−1,6辛二烯 9.87 722 0.02 十一酸 21.94 881 0.30 3−异丙基甲苯 10.00 851 1.79 1,2,4−三甲氧基5−丙烯苯 24.49 741 0.01 1,2,3,4−四甲苯 10.44 915 19.90 十九酸 26.41 732 0.31 2,3−二氢−4−甲基−1H−茚 10.99 859 1.05 5−十二碳烯酸 28.05 773 0.10 2,3−二氢−5−甲基−1H−茚 11.00 777 trace 2−十二酮 28.12 774 0.10 4−异丁基甲苯 11.08 772 0.32 1,2−苯二甲酸二异丁酯 28.48 817 0.19 1−丁基苯 11.23 852 0.30 十五酸 28.50 796 0.27 1S−1,7,7−三甲基−二环[2,2,1]−2−庚酮 11.26 844 0.32 十三酸甲酯 29.82 844 0.18 1,1−二异丁基苯 11.64 819 0.17 11−十六碳烯酸 30.20 871 2.01 1,3−苯二酚单苯甲酸酯 11.68 842 0.09 十八碳烯酸 30.32 776 0.04 辛酸 11.82 799 0.06 十六酸 30.68 890 12.59 4−甲基1−异丙基−3−环己烯醇 12.15 831 0.18 1,11−十二碳二烯 33.77 844 0.08 五甲苯 12.81 770 0.22 1,10−十一碳二烯 33.77 806 0.14 2,3−二氢−4,7−二甲基−1H−茚 13.99 873 0.11 3−丁基−4−乙烯基−环戊烯 33.77 779 0.09 2,3−二氢−4,6−二甲基−1H−茚 14.32 810 0.06 9,12−十八碳二烯酸 33.78 803 0.02 壬酸 14.49 733 0.13 9,12,15−十八碳三烯酸 33.88 807 1.18 5−异丙基−1,3−二甲苯 14.78 795 0.27 十八烯酸 33.96 883 0.01 总计 97.08 表 2 板蓝根挥发性成分的还原能力 (平均值 ± 标准差,n = 3)
Table 2. Reducing activity of volatile substances from Isatidis Radix (mean ± SD, n = 3)
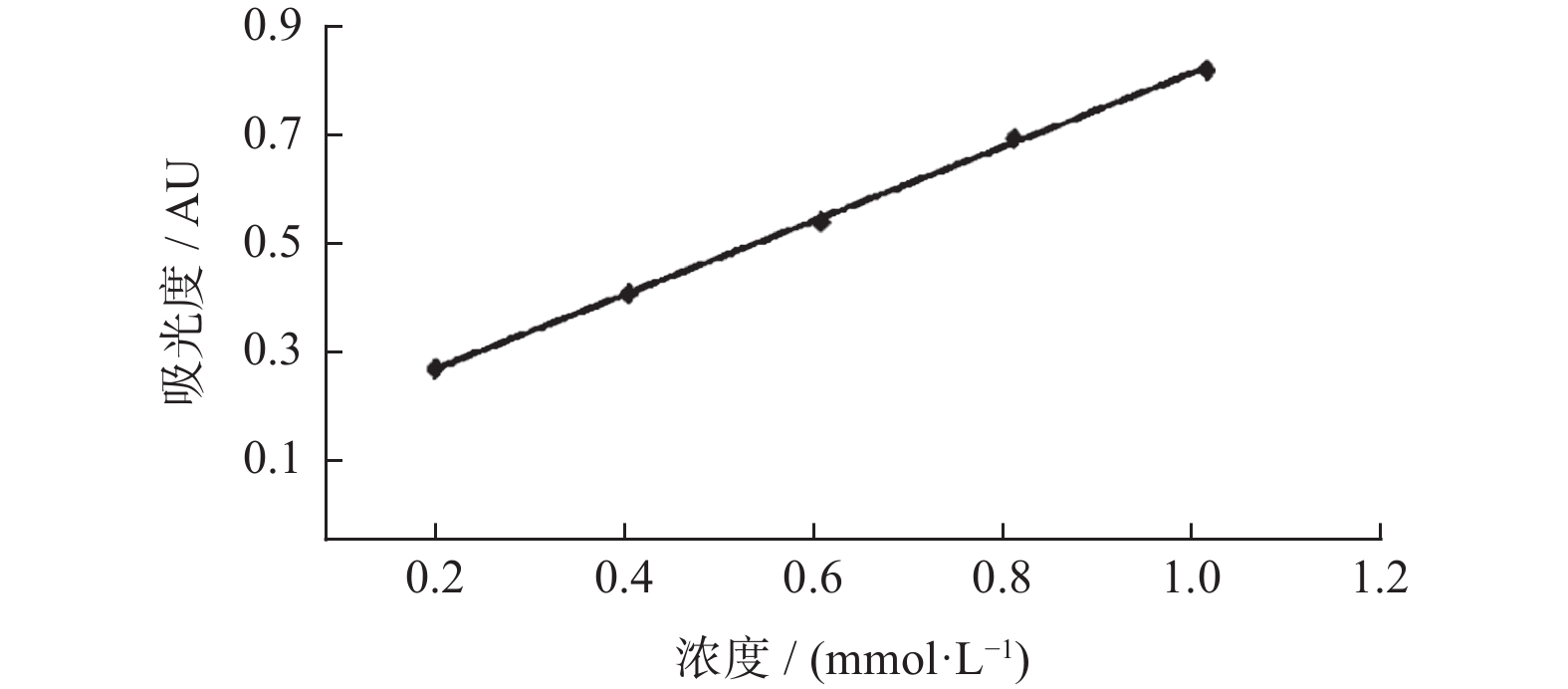
质量浓度
/(mg·mL−1)还原能力 吸光度(普鲁士蓝法)/AU Fe2+(FRAP法)/(mmol·L−1) 0.01 0.058 ± 0.009 — 0.05 0.065 ± 0.020 — 0.25 0.139 ± 0.018 — 1.25 0.418 ± 0.044 0.094 ± 0.033 6.25 0.587 ± 0.377 0.410 ± 0.040 12.50 — 0.915 ± 0.204 25.00 — 1.401 ± 0.130 50.00 — 1.517 ± 0.308 -
[1] CHEN J, ZHU Z, GAO T, et al. Isatidis Radix and isatidis folium: A systematic review on ethnopharmacology, phytochemistry and pharmacology[J] . Journal of Ethnopharmacology,2021, 283:114648. [2] ZHOU W, ZHANG X Y. Research progress of Chinese herbal medicine Radix isatidis (banlangen)[J] . The American Journal of Chinese Medicine,2013,41(4):743 − 764. doi: 10.1142/S0192415X1350050X [3] 何立巍, 李祥, 陈建伟, 等. 板蓝根水提取液中的化学成分[J] . 药学学报,2006,41(12):1193 − 1196. doi: 10.3321/j.issn:0513-4870.2006.12.015 [4] 国家药典委员会. 中华人民共和国药典: 一部[S]. 北京: 中国医药科技出版社, 2020. [5] National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disc susceptibility tests: M2-A2 [S]. Villanova: National Committee for Clinical Laboratory Standards, 1993. [6] DORMAN H J, KOŞAR M, KAHLOS K, et al. Antioxidant properties and composition of aqueous extracts from Mentha species, hybrids, varieties, and cultivars[J] . Journal of Agricultural and Food Chemistry,2003,51(16):4563 − 4569. doi: 10.1021/jf034108k [7] BENZIE I F, STRAIN J J. The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": the FRAP assay[J] . Analytical Biochemistry,1996,239(1):70 − 76. [8] BLOIS M S. Antioxidant determinations by the use of stable free radical[J] . Nature,1958,181(4617):1199 − 1200. doi: 10.1038/1811199a0 [9] 徐红颖, 禹晓梅, 梁逸曾, 等. 板蓝根挥发性成分GC-MS分析[J] . 天然产物研究与开发,2008,20(2):292 − 294. doi: 10.3969/j.issn.1001-6880.2008.02.025 [10] 孔维军, 赵雅玲, 山丽梅, 等. 微量热法研究板蓝根中四种有机酸对微生物生长代谢的影响[J] . 生物工程学报,2008,24(4):646 − 650. doi: 10.3321/j.issn:1000-3061.2008.04.020 [11] KONG W, ZHAO Y, SHAN L, et al. Thermochemical studies on the quantity-antibacterial effect relationship of four organic acids from Radix Isatidis on Escherichia coli growth[J] . Biological and Pharmaceutical Bulletin,2008,31(7):1301 − 1305. doi: 10.1248/bpb.31.1301 [12] XIAO P, HUANG H, CHEN J, et al. In vitro antioxidant and anti-inflammatory activities of Radix Isatidis extract and bioaccessibility of six bioactive compounds after simulated gastro-intestinal digestion[J] . Journal of Ethnopharmacology,2014,157:55 − 61. doi: 10.1016/j.jep.2014.09.005 [13] 赵琳静, 李洪森, 吴晓英, 等. 板蓝根抗氧化成分的提取及活性分析[J] . 食品工业科技,2014,(10):195 − 197,201. [14] AMORATI R, FOTI M C, VALGIMIGLI L. Antioxidant activity of essential oils[J] . Journal of Agricultural and Food Chemistry,2013,61(46):10835 − 10847. doi: 10.1021/jf403496k [15] CEDROWSKI J, DĄBROWA K, PRZYBYLSKI P, et al. Antioxidant activity of two edible isothiocyanates: Sulforaphane and erucin is due to their thermal decomposition to sulfenic acids and methylsulfinyl radicals[J] . Food Chemistry,2021,353:129213. doi: 10.1016/j.foodchem.2021.129213 -






 下载:
下载: