A N-phenylnaphthalimide derivative with aggregation-induced emission for cell imaging
-
摘要:
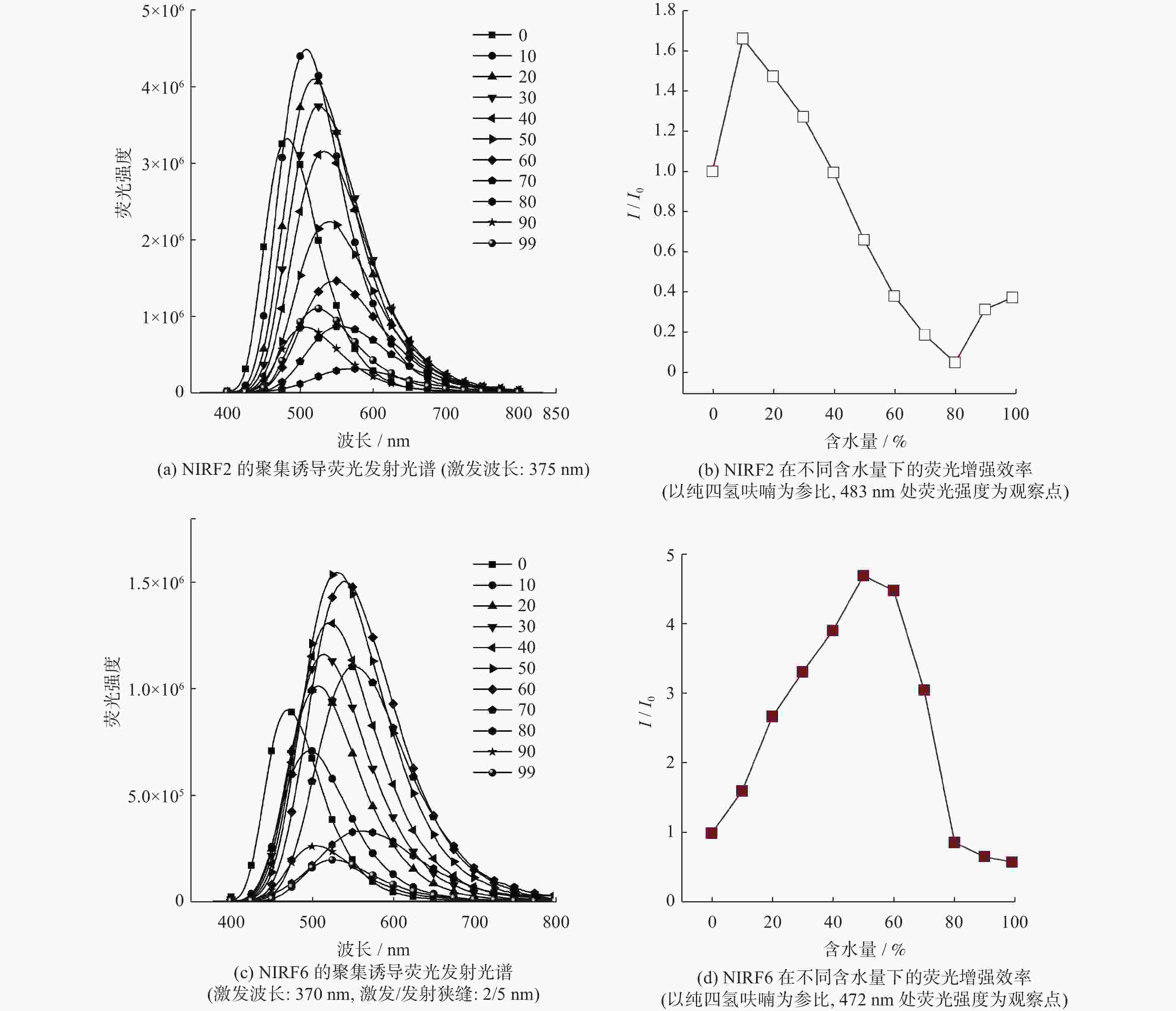
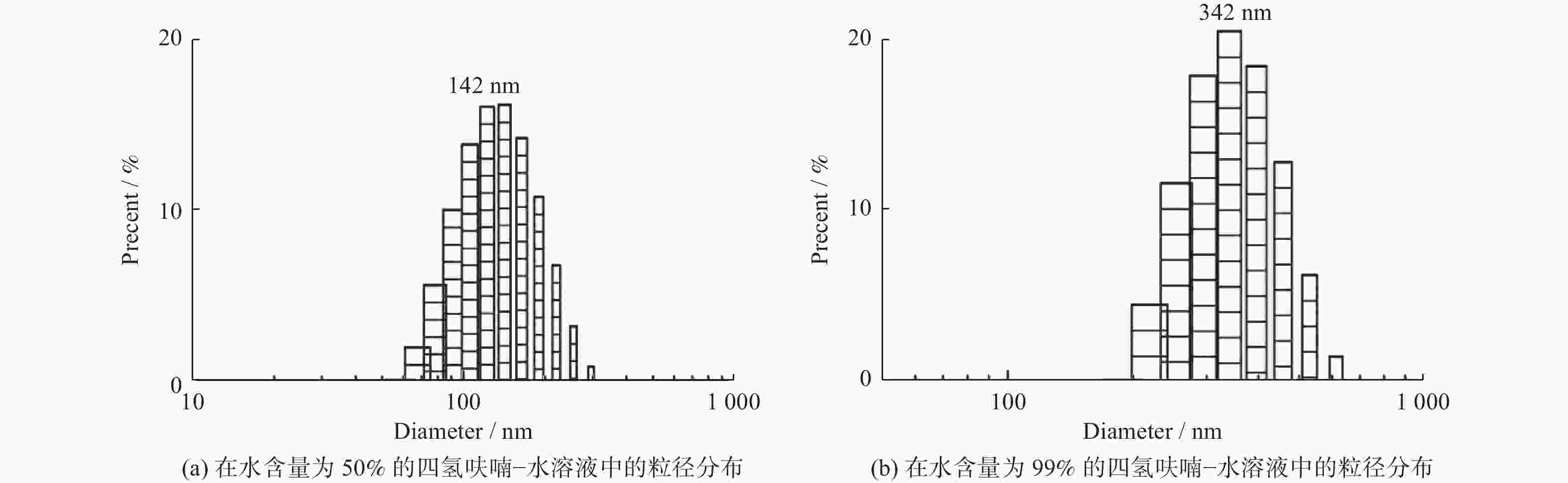
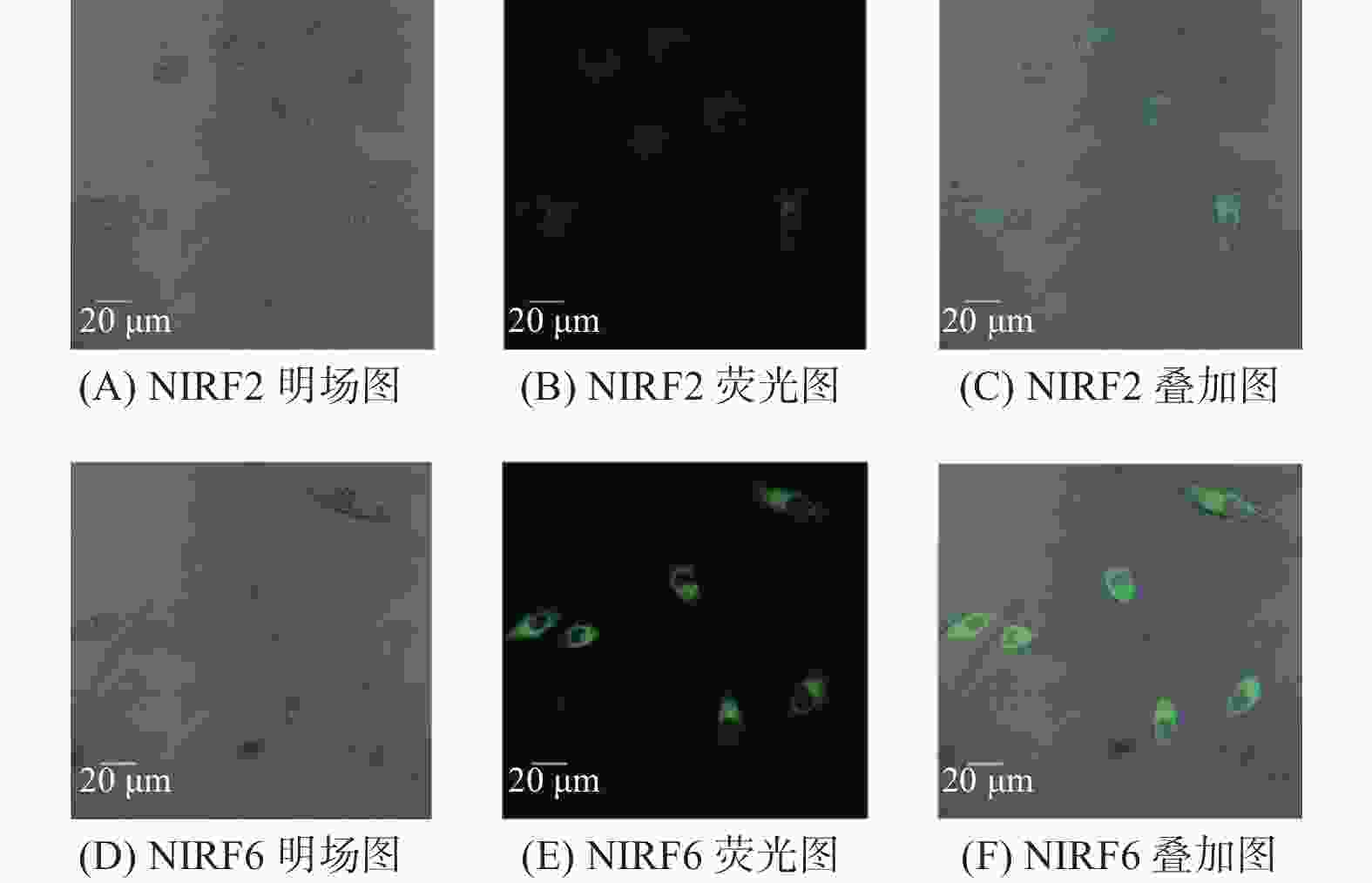
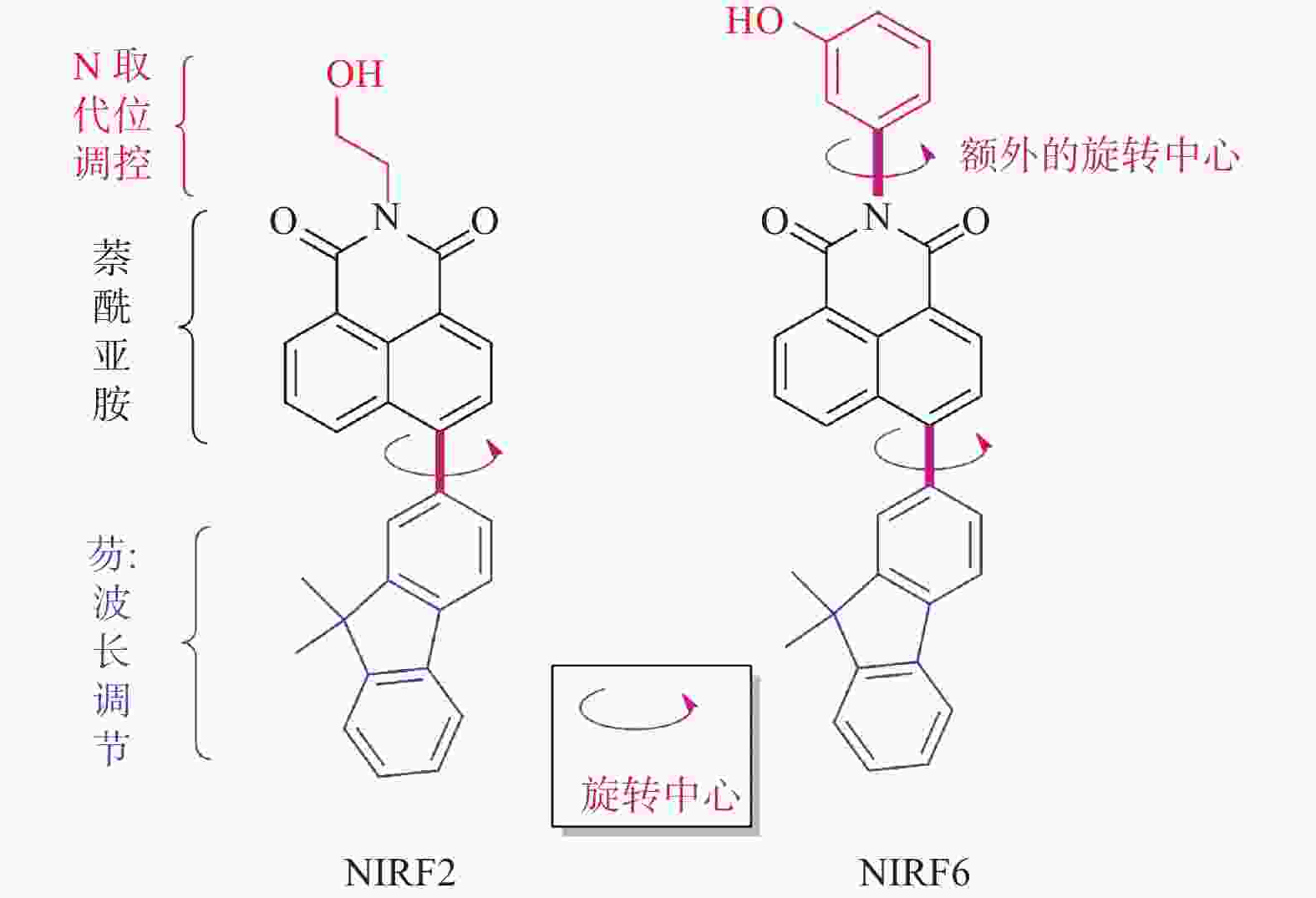
聚集诱导发光分子在有机光电功能材料领域有着重要的应用价值. 利用常见的ACQ−活性萘酰亚胺染料, 通过典型的铃木偶联反应,在萘酰亚胺荧光团的4位引入一个去平面化苯环,设计并合成两个新的聚集诱导发光(Aggregation-Induced Emission, AIE)分子NIRF2和NIRF6. 通过1H、13C NMR和HRMS谱对其进行结构表征. 通过紫外−可见光谱、荧光发射光谱和动态激光散射测试研究二者结构与聚集诱导发光的构效关系. 最后,NIRF2和NIRF6成功应用于Hela细胞的细胞质成像. 研究表明,提出的优化设计策略可以实现萘酰亚胺基AIE活性分子的构建.
Abstract:Aggregation-induced emission (AIE)-active materials show important applications in organic photoelectric functional materials. By introducing a phenyl ring, a deplanarization core into the 4-site of naphthalimide chromophore through the typical Suzuki coupling reaction, two new AIE molecules (NIRF2 and NIRF6) based on the common ACQ-active naphthalimide dye were designed and synthesized. Their structures were fine characterized by 1H & 13C NMR and HRMS spectra. The structure-properties relationships were discussed by UV-vis and fluorescent spectra and dynamic laser scattering tests. Finally, both NIRF2 and NIRF6 were successfully applied to image the cytoplasm of the Hela cells. The study results show that the optimized designing strategy can achive the naphthalimide-based AIE-active molecules.
-
Key words:
- naphthalimide /
- aggregation-induced emission (AIE) /
- cell imaging
-
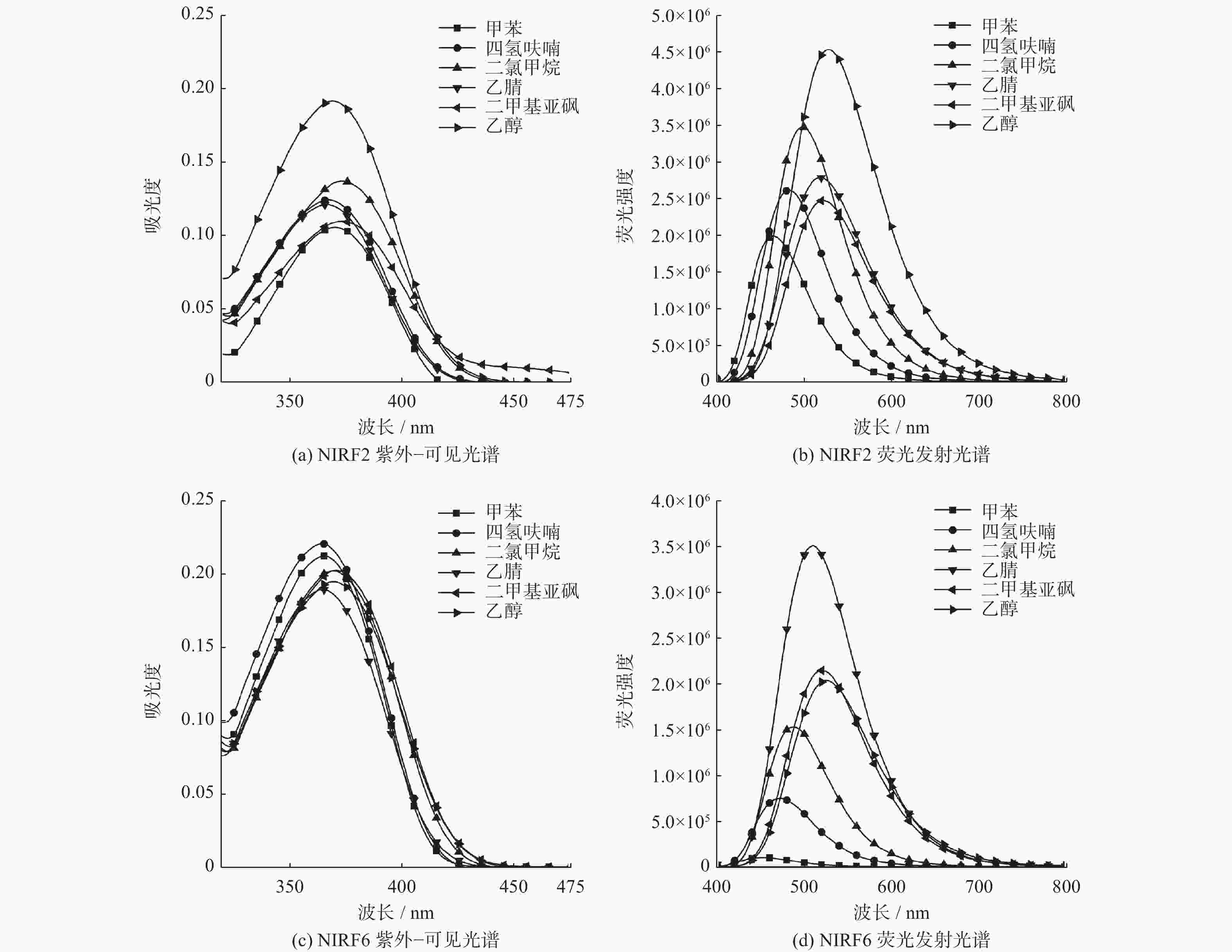
表 1 化合物NIRF2与NIRF6在不同溶剂中的光学性质
Table 1. Optical properties of compounds NIRF2 and NIRF6 in different solvents
化合物 溶剂 吸收波长/nm 发射波长/nm 摩尔消光系数 Stokes 位移/nm NIRF2 甲苯 369 464 10500 95 四氢呋喃 368 484 12400 116 二氯甲烷 374 499 13700 125 乙腈 366 519 12100 153 二甲基亚砜 375 522 10900 147 乙醇 369 529 19200 160 NIRF6 甲苯 366 449 21200 83 四氢呋喃 365 470 22000 105 二氯甲烷 371 487 20200 116 乙腈 366 510 19000 144 二甲基亚砜 372 521 20200 149 乙醇 370 526 19400 156 -
[1] LI R J, HU W P, LIU Y Q, et al. Micro- and nanocrystals of organic semiconductors[J] . Accounts of Chemical Research,2010,43(4):529 − 540. doi: 10.1021/ar900228v [2] WANG C L, DONG H L, JIANG L, et al. Organic semiconductor crystals[J] . Chemical Society Review,2018,47(2):422 − 500. doi: 10.1039/C7CS00490G [3] HEDLEY G J, RUSECKAS A, SAMUEL I D W. Light harvesting for organic photovoltaics[J] . Chemical Reviews,2017,117(2):796 − 837. doi: 10.1021/acs.chemrev.6b00215 [4] LI H, SHI W, SONG J, et al. Chemical and biomolecule sensing with organic field-effect transistors[J] . Chemical Reviews,2019,119(1):3 − 35. doi: 10.1021/acs.chemrev.8b00016 [5] LUO J D, XIE Z L, LAM J W Y, et al. Aggregation-induced emission of 1-methyl-1, 2, 3, 4, 5-pentaphenylsilole[J] . Chemical Communications,2001,37(18):1740 − 1741. [6] LIU X Q, ZHANG K, GAO J F, et. al. Monochromophore-based phosphorescence and fluorescence from pure organic assemblies for ratiometric hypoxia detection[J] . Angewandte Chemie International Edition,2020,59(52):23456 − 23460. doi: 10.1002/anie.202007039 [7] ZHENG X J, ZHU W C, NI F, et al. Simultaneous dual-colour tracking lipid droplets and lysosomes dynamics using a fluorescent probe[J] . Chemical Science,2019,10(8):2342 − 2348. doi: 10.1039/C8SC04462G [8] LOVING G, IMPERIALI B. A versatile amino acid analogue of the solvatochromic fluorophore 4-N,N-dimethylamino-1, 8-naphthalimide: A powerful tool for the study of dynamic protein interactions[J] . Journal of the American Chemical Society,2008,130(41):13630 − 13638. doi: 10.1021/ja804754y [9] HU R R, LEUNG N L C, TANG B Z. AIE macromolecules: Syntheses, structures and functionalities[J] . Chemical Society Review,2014,43(13):4494 − 4562. doi: 10.1039/C4CS00044G [10] LI J S, SHEN P C, ZHAO Z, et. al. Through-space conjugation: A thriving alternative for optoelectronic materials[J] . CCS Chemistry,2019,1(2):181 − 196. doi: 10.31635/ccschem.019.20180020 [11] ZHANG H K, ZHAO Z, TURLEY A T, et al. Aggregate science: From structures to properties[J] . Advanced Materials,2020,32(36):2001457. [12] MUKHERJEE S, THILAGAR P. Insights into the AIEE of 1, 8-naphthalimides (NPIs): Inverse effects of intermolecular interactions in solution and aggregates[J] . Chemistry: A European Journal,2014,20(26):8012 − 8023. [13] GOPIKRISHNA P, MEHER N, IYER P K. Functional 1, 8-naphthalimide AIE/AIEEgens: Recent advances and prospects[J] . ACS Applied Materials & Interfaces,2018,10(15):12081 − 12111. [14] BALACHANDRA C, GOVINDARAJU T. Cyclic dipeptide-guided aggregation-induced emission of naphthalimide and its application for the detection of phenolic drugs[J] . The Journal of Organic Chemistry,2020,85(3):1525 − 1536. doi: 10.1021/acs.joc.9b02580 [15] DELENTE J M, UMADEVI D, SHANMUGARAJU S, et al. Aggregation induced emission (AIE) active 4-amino-1, 8-naphthalimide-Tröger's base for the selective sensing of chemical explosives in competitive aqueous media[J] . Chemical Communications,2020,56(17):2562 − 2565. doi: 10.1039/C9CC08457F [16] QI Q K, HUANG L, YANG R Q, et al. Rhodamine-naphthalimide demonstrated a distinct aggregation-induced emission mechanism: Elimination of dark-states via dimer interactions (EDDI)[J] . Chemical Communications,2019,55(10):1446 − 1449. doi: 10.1039/C8CC09212E [17] LIU X G, QIAO Q L, TIAN W M, et al. Aziridinyl fluorophores demonstrate bright fluorescence and superior photostability by effectively inhibiting twisted intramolecular charge transfer[J] . Journal of the American Chemical Society,2016,138(22):6960 − 6963. doi: 10.1021/jacs.6b03924 -





 下载:
下载: