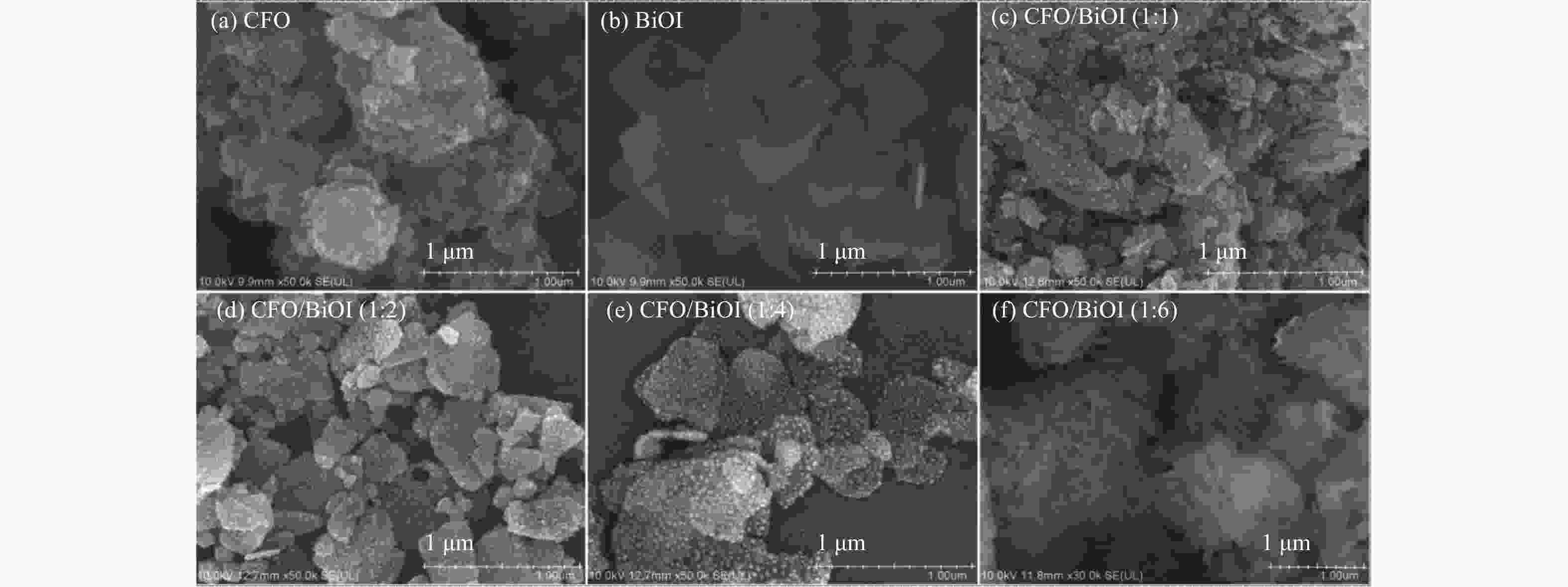
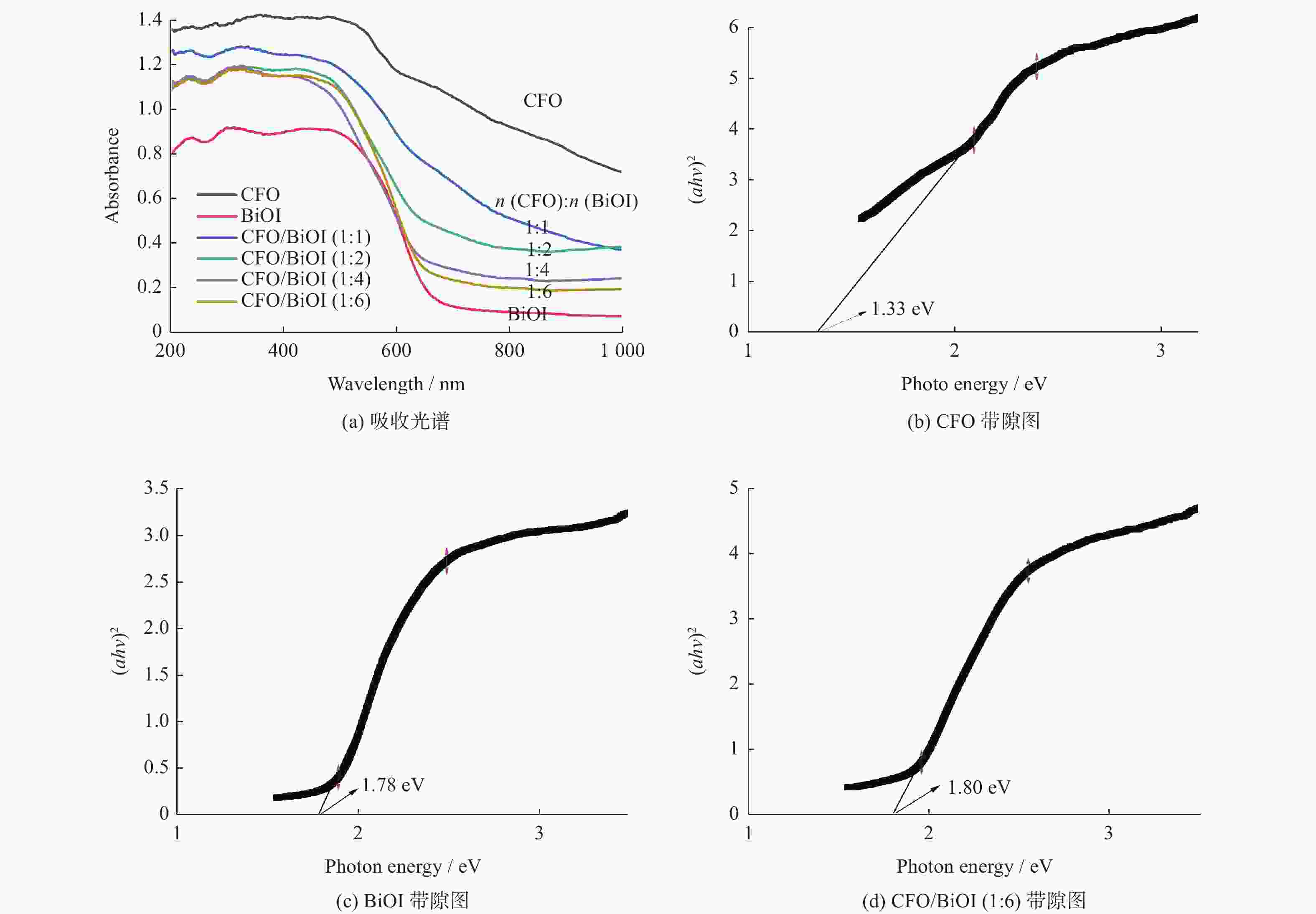
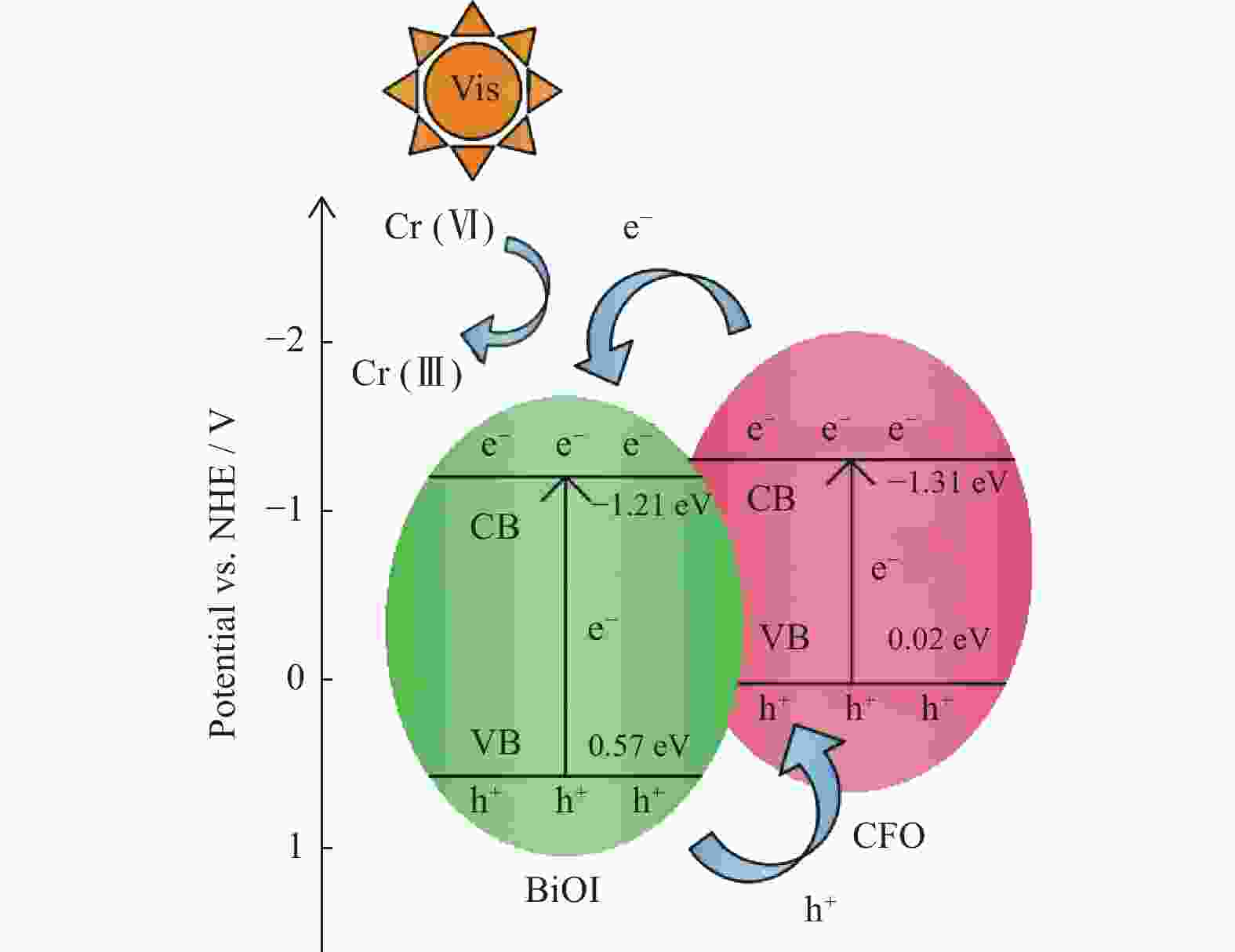
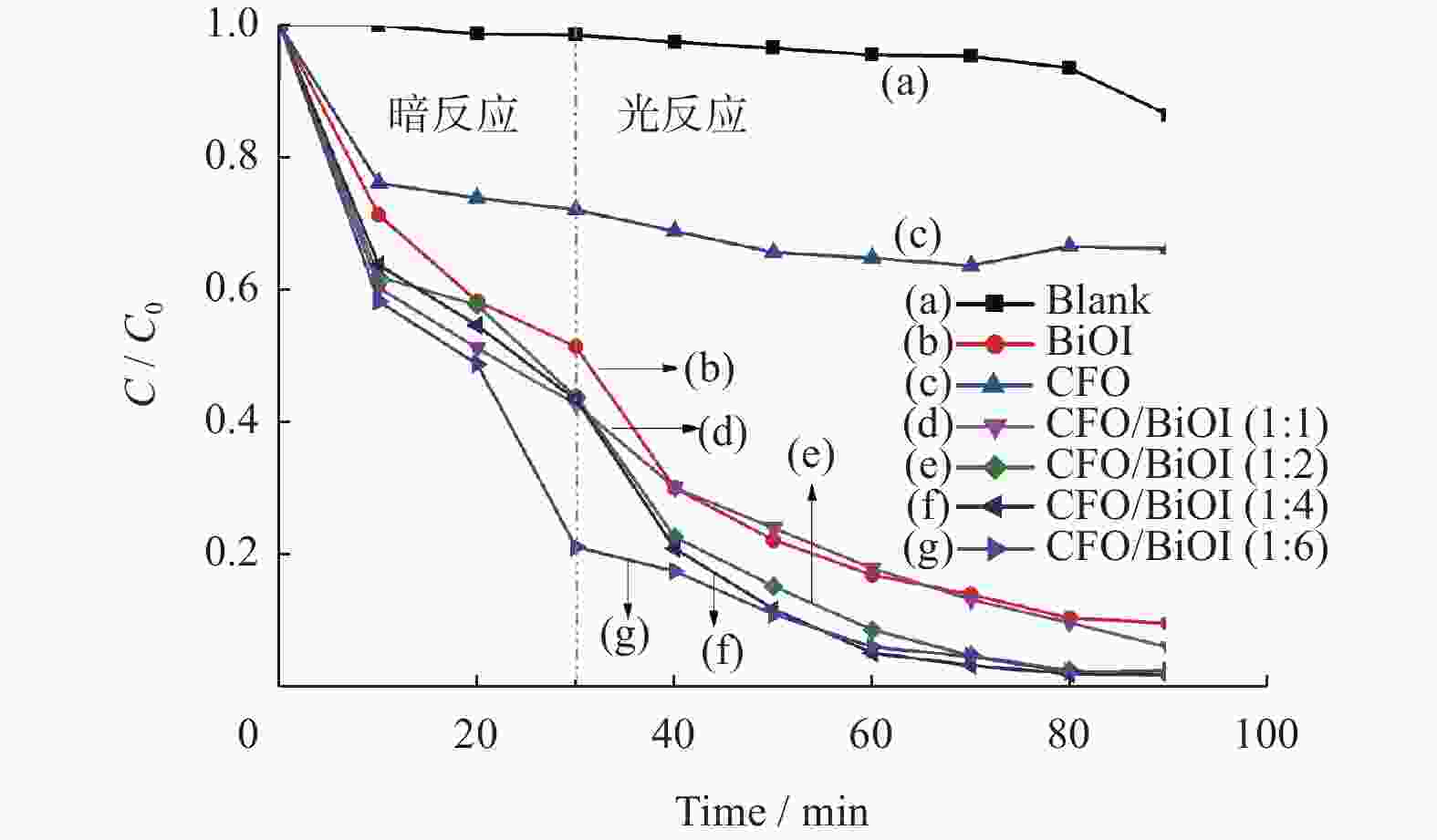
Preparation of CoFe2O4/BiOI magnetic photocatalyst and its application in Cr(VI) removal
-
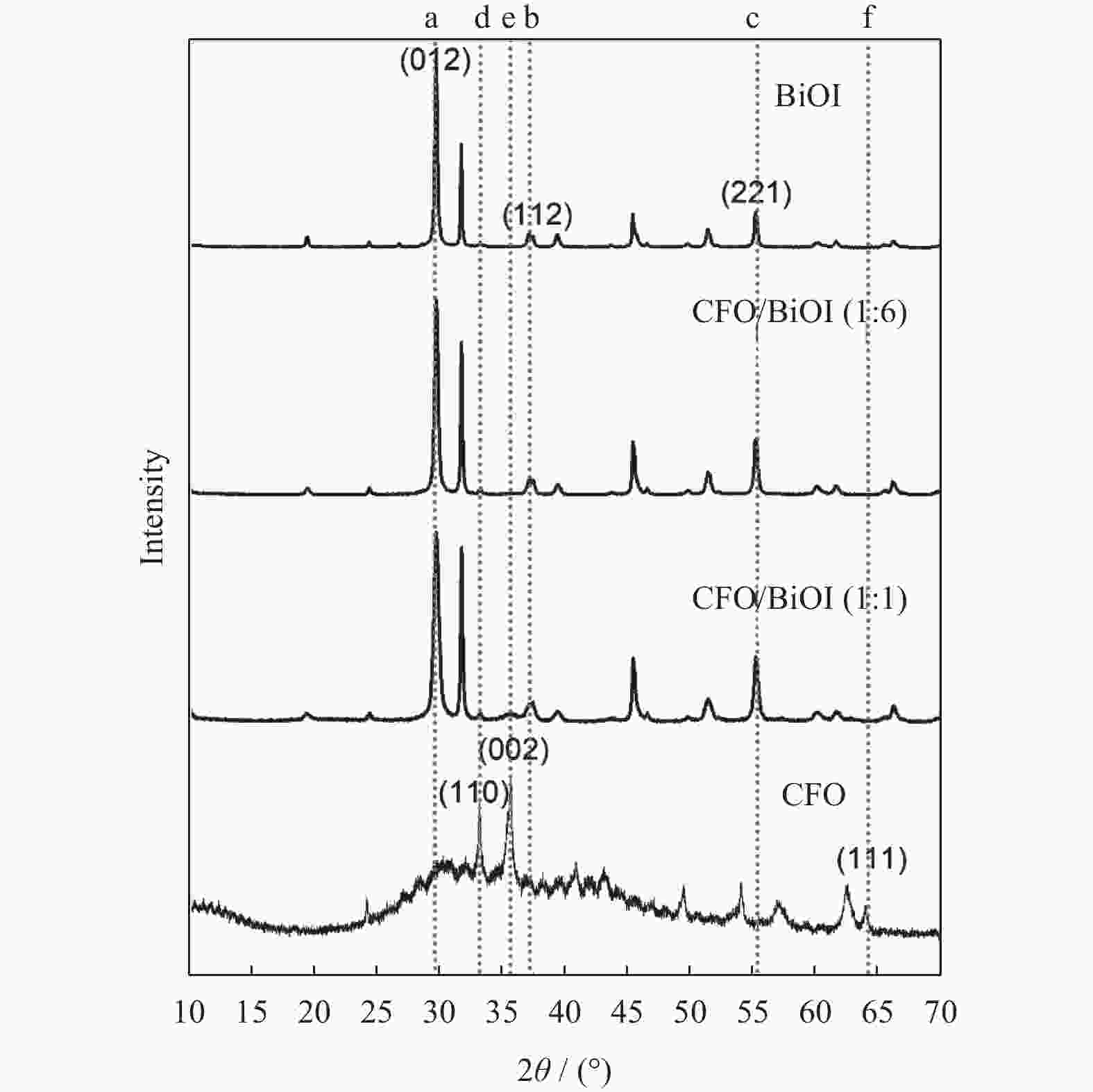
摘要: 水体中的重金属六价铬离子对人体、环境均有严重影响,其含量是水体质量优劣的重要指标之一,需要对其进行严格控制. 以BiOI为光催化剂,CoFe2O4(简称CFO)作为复合材料的磁性成分,合成CFO/BiOI纳米磁性光催化材料,研究不同摩尔比的CFO/BiOI对六价铬去除性能的影响. 研究发现,CFO/BiOI复合材料在六价铬催化去除中,表现出比纯CFO和BiOI更好的性能. 此外,CFO的引入赋予了材料磁性,可将催化材料通过磁力快速分离.Abstract: The heavy metal hexavalent chromium (Cr(VI)) ion in water has serious impact on human body and environment, and its concentration is one of the important indicators of water quality, which needs to be strictly controlled. CFO/BiOI nanometer magnetic photocatalyst material was synthesized with BiOI as photocatalyst and CoFe2O4 (CFO for short) as magnetic component of composite material, and the effect of different proportion of CFO/BiOI on the removal performance of hexavalent chromium was studied. It was found that the CFO/BiOI composite exhibited better catalytic performance than pure CFO and BiOI in the application of hexavalent chromium removal. This may be caused by the construction of heterojunction between CFO and BiOI, which accelerated the charge separation. In addition, the introduction of CFO endowed the BiOI material with magnetism, which allowed rapidly separation of the catalyst through magnetic force.
-
表 1 不同比例CFO/BiOI(n(CFO)∶n(BiOI) = 1∶1~1∶6)所用物质及使用量
Table 1. Materials and amounts used for CFO/BiOI with different ratios (n(CFO)∶n(BiOI) = 1∶1~1∶6)
药品用量 m(CFO)
/gm (KI)
/gm (Bi(NO3)3·5H2O)
/gV(EG·(CH2OH)2)
/mLCFO/BiOI(1∶1) 0.2815 0.1992 0.5821 10 CFO/BiOI(1∶2) 0.1408 0.1992 0.5821 10 CFO/BiOI(1∶4) 0.0704 0.1992 0.5821 10 CFO/BiOI(1∶6) 0.0469 0.1992 0.5821 10 -
[1] 张汉池, 张继军, 刘峰. 铬的危害与防治[J] . 内蒙古石油化工,2004(1):72 − 73. doi: 10.3969/j.issn.1006-7981.2004.01.035 [2] 梁奇峰. 铬与人体健康[J] . 广东微量元素科学,2006(2):67 − 69. doi: 10.3969/j.issn.1006-446X.2006.02.018 [3] 谢文强. 六价铬对人体急性与慢性危害探究[J] . 资源节约与环保,2016(7):131,135. [4] 李爱琴, 唐宏建, 王阳峰. 环境中铬污染的生态效应及其防治[J] . 中国环境管理干部学院学报,2006(1):74 − 77. doi: 10.3969/j.issn.1008-813X.2006.01.023 [5] 王勇, 刘叶. 水体重金属污染现状及其健康风险评价[J] . 居舍,2018(32):180. [6] THATOI H, DAS S, MISHRA J, et al. Bacterial chromate reductase, a potential enzyme for bioremediation of hexavalent chromium: A review[J] . Journal of Environmental Management,2014,146:383 − 399. doi: 10.1016/j.jenvman.2014.07.014 [7] YANG J K, LEE S M, FARROKHI M, et al. Photocatalytic removal of Cr(VI) with illuminated TiO2[J] . Desalination and Water Treatment,2012,46(1/2/3):375 − 380. doi: 10.1080/19443994.2012.677564 [8] 周祥博, 刘亲壮, 朱光平, 等. 镁铝双氢氧化物和镁铝双氢氧化物负载型高岭土对水体中Cr(Ⅵ)的吸附性能研究[J] . 淮北师范大学学报(自然科学版),2016,37(1):17 − 21. [9] YI Y H, LV J L, LIU Y, et al. Synthesis and application of modified Litchi peel for removal of hexavalent chromium from aqueous solutions[J] . Journal of Molecular Liquids,2017,225:28 − 33. doi: 10.1016/j.molliq.2016.10.140 [10] DENG L, SHI Z, WANG L, et al. Fabrication of a novel NiFe2O4 /Zn-Al layered double hydroxide intercalated with EDTA composite and its adsorption behavior for Cr(VI) from aqueous solution[J] . Journal of Physics and Chemistry of Solids,2017,104:79 − 90. doi: 10.1016/j.jpcs.2016.12.030 [11] SHEN C S, CHEN H, WU S S, et al. Highly efficient detoxification of Cr(VI) by chitosan-Fe(III) complex: Process and mechanism studies[J] . Journal of Hazardous Materials,2013,244/245:689 − 697. doi: 10.1016/j.jhazmat.2012.10.061 [12] 李伟. Fe(Ⅱ)掺杂TiO2光催化剂处理电镀废水中的六价铬[J] . 电镀与涂饰,2021,40(2):124 − 128. [13] 赵海亮. g−C3N4/CuS复合光催化剂的制备、表征及其光催化去除甲基橙和Cr(Ⅵ)性能研究[D]. 昆明: 昆明理工大学, 2017. [14] LI M Y, ZHANG G X, FENG C Q, et al. Highly sensitive detection of chromium (VI) by photoelectrochemical sensor under visible light based on Bi SPR-promoted BiPO4/BiOI heterojunction[J] . Sensors and Actuators B: Chemical,2020,305:127449. doi: 10.1016/j.snb.2019.127449 [15] LI M Y, HE R, WANG S Q, et al. Visible light driven photoelectrochemical sensor for chromium(VI) by using BiOI microspheres decorated with metallic bismuth[J] . Microchimica Acta,2019,186(6):345. doi: 10.1007/s00604-019-3463-0 [16] CHENG D, WU H M, FENG C Q, et al. Highly sensitive detection of chromium (VI) by photoelectrochemical sensor based on p-n heterojunction of carbon nitride-modified BiOI[J] . Journal of Alloys and Compounds,2021,882:160690. doi: 10.1016/j.jallcom.2021.160690 [17] KIVYIRO A O, DARKWAH W K, BOFAH-BUOH R, et al. Photocatalytic reduction of hexavalent chromium (Cr6+) over BiOI calcined at different temperature under visible light irradiation[J] . Chemistryselect,2021,6(24):5906 − 5916. doi: 10.1002/slct.202101285 [18] 李双芝, 赵卿, 刘丽君, 等. BiOI/g−C3N4协同光催化还原Cr(Ⅵ)及光降解罗丹明B[J] . 武汉纺织大学学报,2017,30(3):64 − 68. doi: 10.3969/j.issn.2095-414X.2017.03.014 [19] 任春溶. 磁性纳米复合材料的制备及其对重金属离子的吸附性能研究[D]. 杭州: 浙江大学, 2017. [20] 王力霞. 铁磁性金属、铁氧体及其复合物的合成与应用研究[D]. 长春: 吉林大学, 2013. [21] ZHANG H Q, YANG J X, GUO L, et al. Microwave-aided synthesis of BiOI/g−C3N4 composites and their enhanced catalytic activities for Cr(VI) removal[J] . Chemical Physics Letters,2021,762:138143. doi: 10.1016/j.cplett.2020.138143 [22] FU Z T, HUANG C S, ZHAO X, et al. Study on preparation and recovery of magnetic BiOI/rGO/Fe3O4 composite photocatalyst[J] . Results in Physics,2020,16:102931. doi: 10.1016/j.rinp.2020.102931 [23] ZOU B J, CHANG X J, YANG J X, et al. Plasma treated h−BN nanoflakes as barriers to enhance anticorrosion of acrylic coating on steel[J] . Progress in Organic Coatings,2019,133:139 − 144. doi: 10.1016/j.porgcoat.2019.04.040 [24] YANG J X, ZHANG J J, ZOU B J, et al. Black SnO2-TiO2 nanocomposites with high dispersion for photocatalytic and photovoltalic applications[J] . ACS Applied Nano Materials,2020,3(5):4265 − 4273. doi: 10.1021/acsanm.0c00432 [25] PENG S S, YANG J X, GUO L, et al. Shape-dependent CeO2@BiOI for degradation of aqueous Cr(VI)[J] . Advanced Materials Interfaces,2020,7(9):1 − 9. [26] YAN C X, ZHANG Z L, WANG W J, et al. Synthesis and characterization of polyaniline-modified BiOI: A visible-light-response photocatalyst[J] . Journal of Materials Science: Materials in Electronics,2018,29(21):18343 − 18351. doi: 10.1007/s10854-018-9948-5 [27] JIAO H P, YU X, LIU Z Q, et al. One-pot synthesis of heterostructured Bi2S3/BiOBr microspheres with highly efficient visible light photocatalytic performance[J] . Rsc Advances,2015,5(21):16239 − 16249. doi: 10.1039/C4RA16948D [28] LI F T, WANG Q, RAN J R, et al. Ionic liquid self-combustion synthesis of BiOBr/Bi24O31Br10 heterojunctions with exceptional visible-light photocatalytic performances[J] . Nanoscale,2015,7(3):1116 − 1126. doi: 10.1039/C4NR05451B [29] Wang J L, Yu Y, Zhang L Z. Highly efficient photocatalytic removal of sodium pentachlorophenate with Bi3O4Br under visible light[J] . Applied Catalysis B: Environmental,2013,136/137:112 − 121. doi: 10.1016/j.apcatb.2013.02.009 [30] XU Y, SCHOONEN M A A. The absolute energy positions of conduction and valence bands of selected semiconducting minerals[J] . American Mineralogist,2000,85(3/4):543 − 556. doi: 10.2138/am-2000-0416 [31] CHENG H F, HUANG B B, QIN X Y, et al. A controlled anion exchange strategy to synthesize Bi2S3 nanocrystals/BiOCl hybrid architectures with efficient visible light photoactivity[J] . Chemical Communications,2012,48(1):97 − 99. doi: 10.1039/C1CC15487G [32] JIANG J, ZHANG X, SUN P B, et al. ZnO/BiOI heterostructures: Photoinduced charge-transfer property and enhanced visible-light photocatalytic activity[J] . The Journal of Physical Chemistry C,2011,115(42):20555 − 20564. doi: 10.1021/jp205925z -





 下载:
下载: