Design and optimization of dimethyl ether reforming reactor for hydrogen production
-
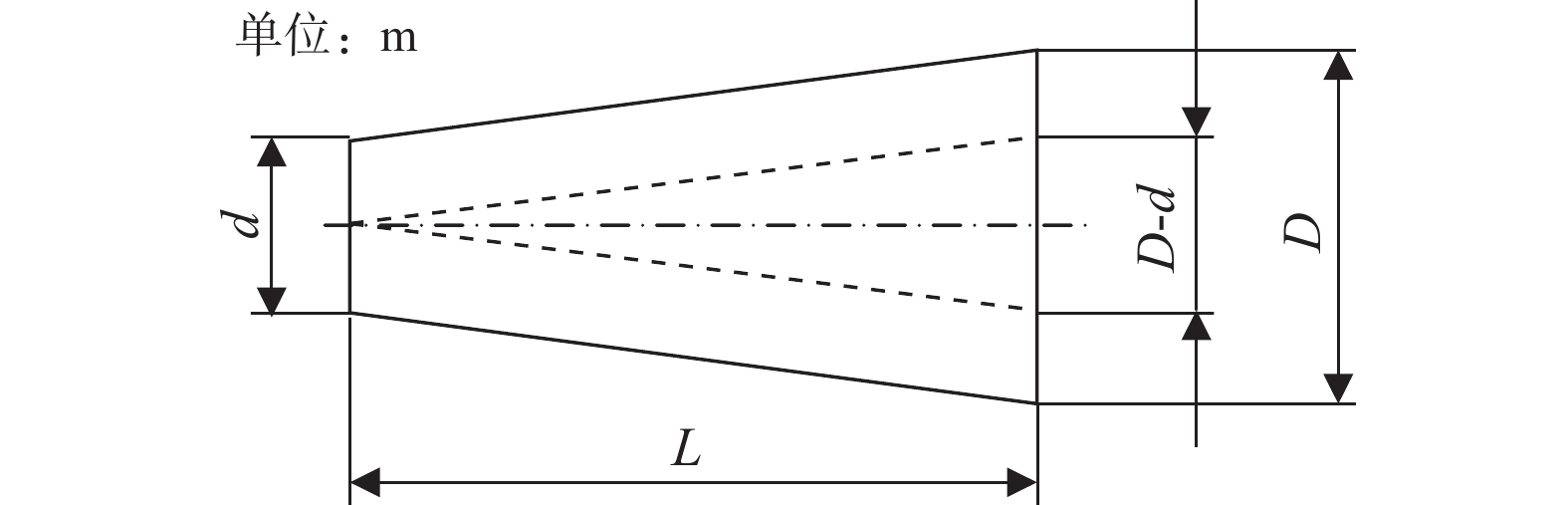
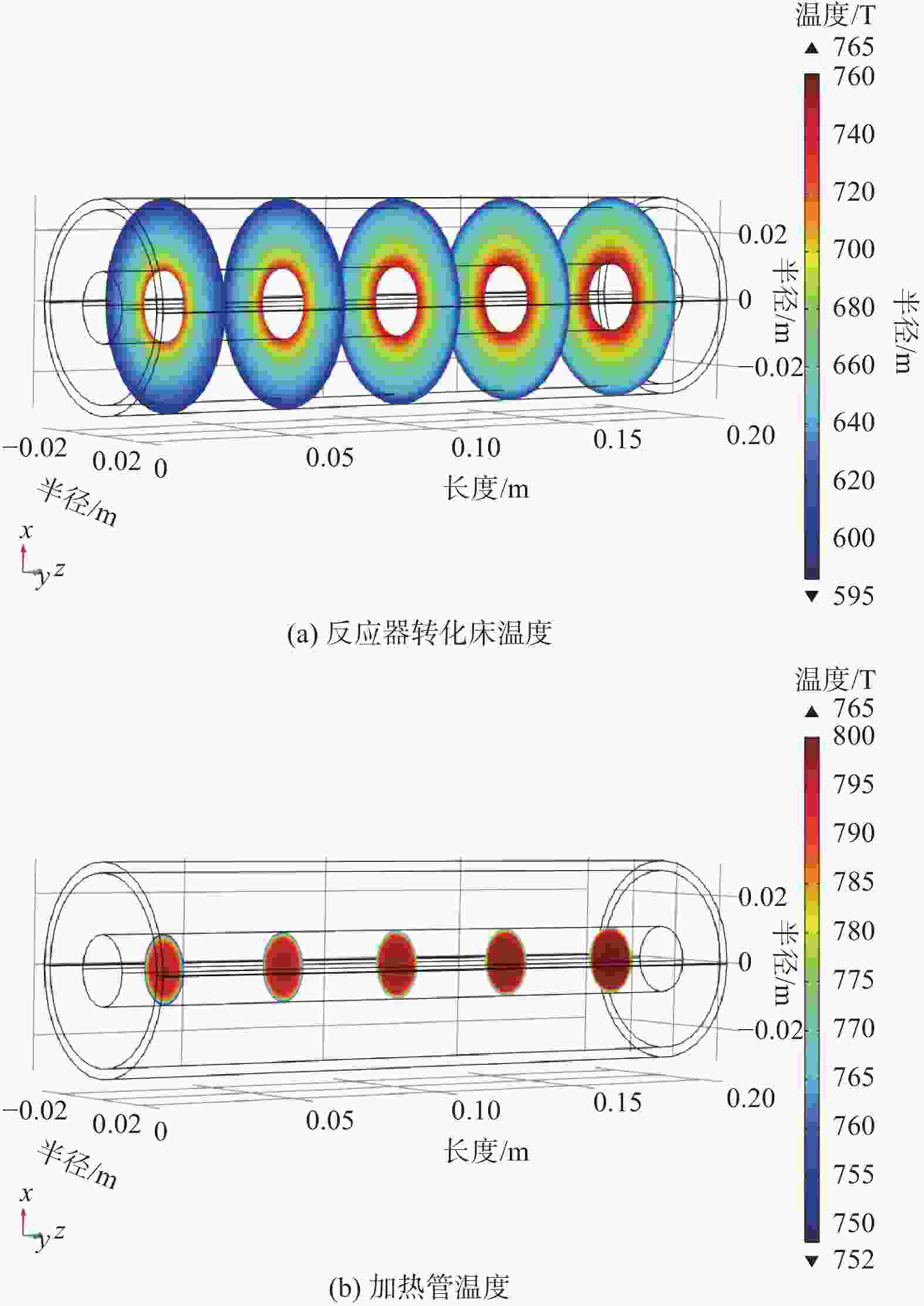
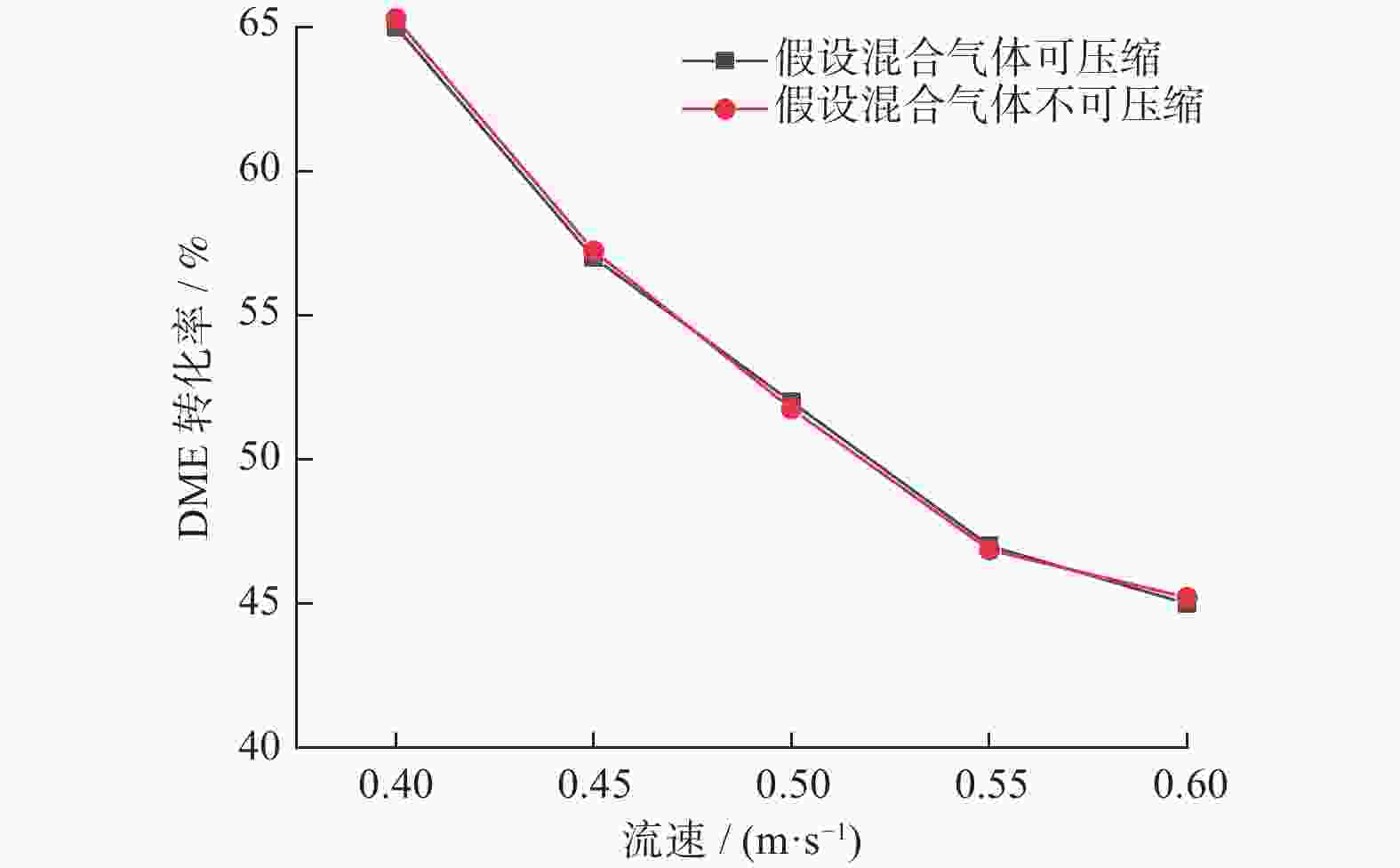
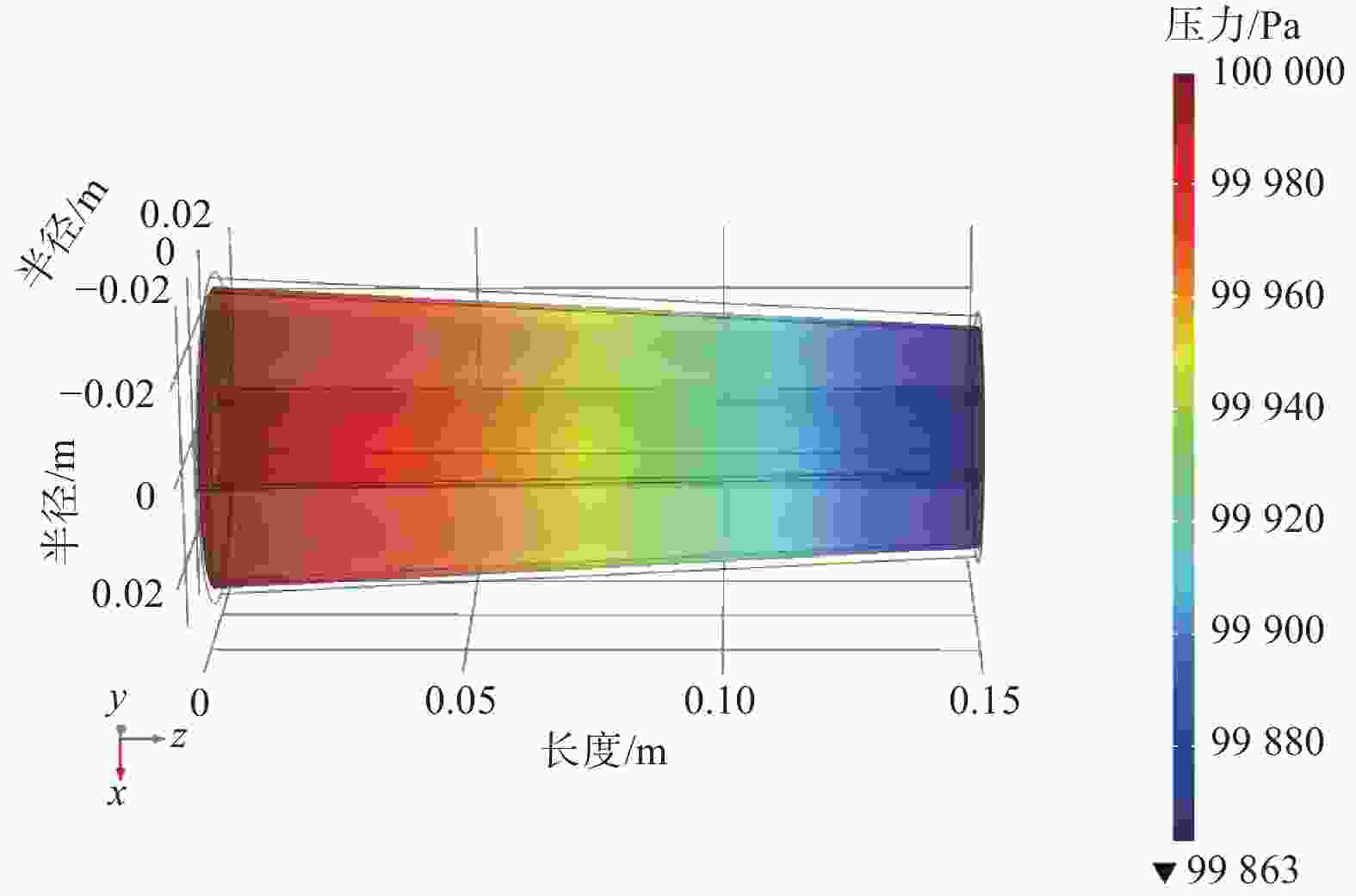
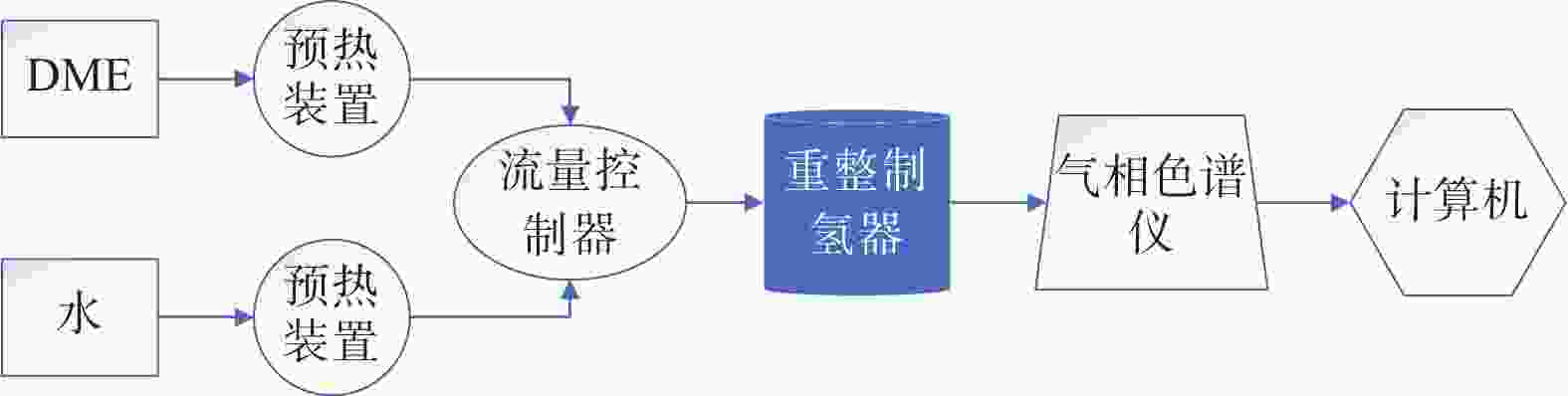
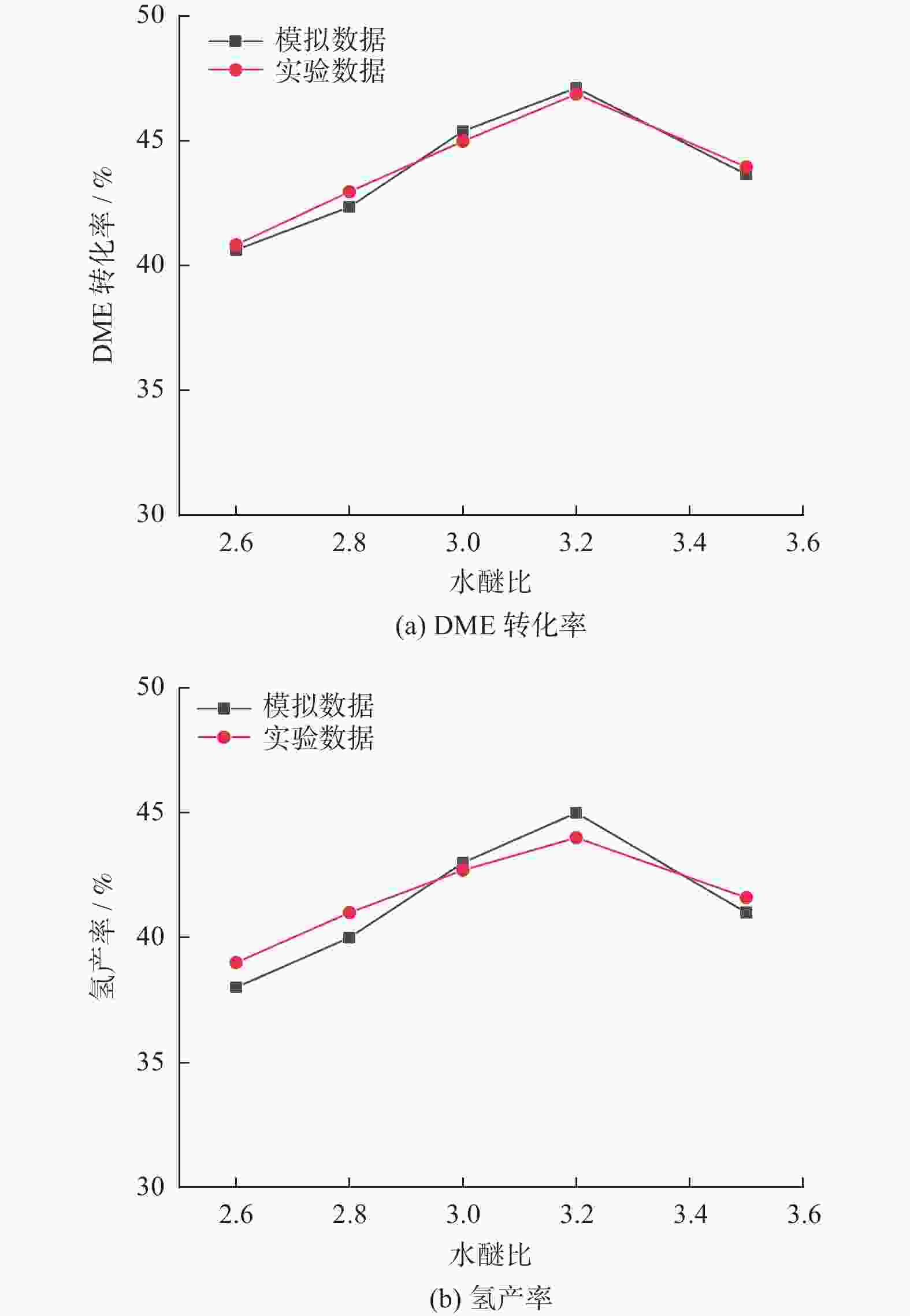
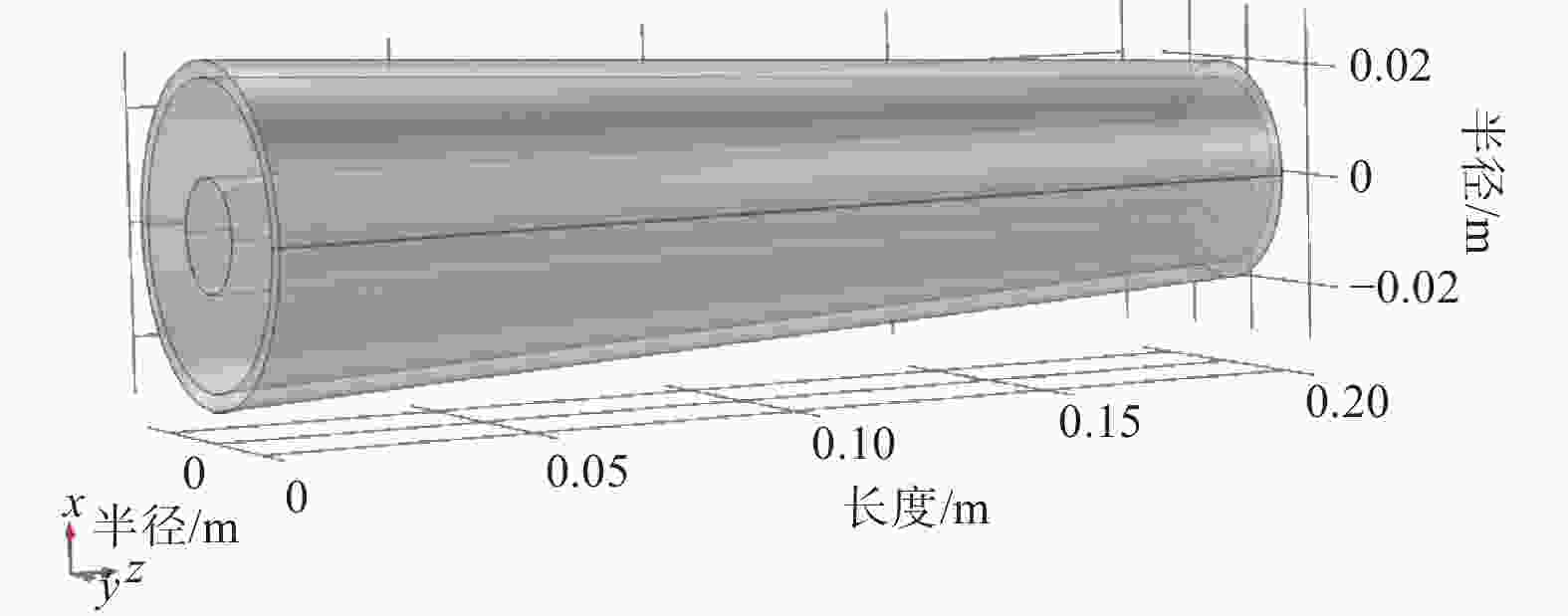
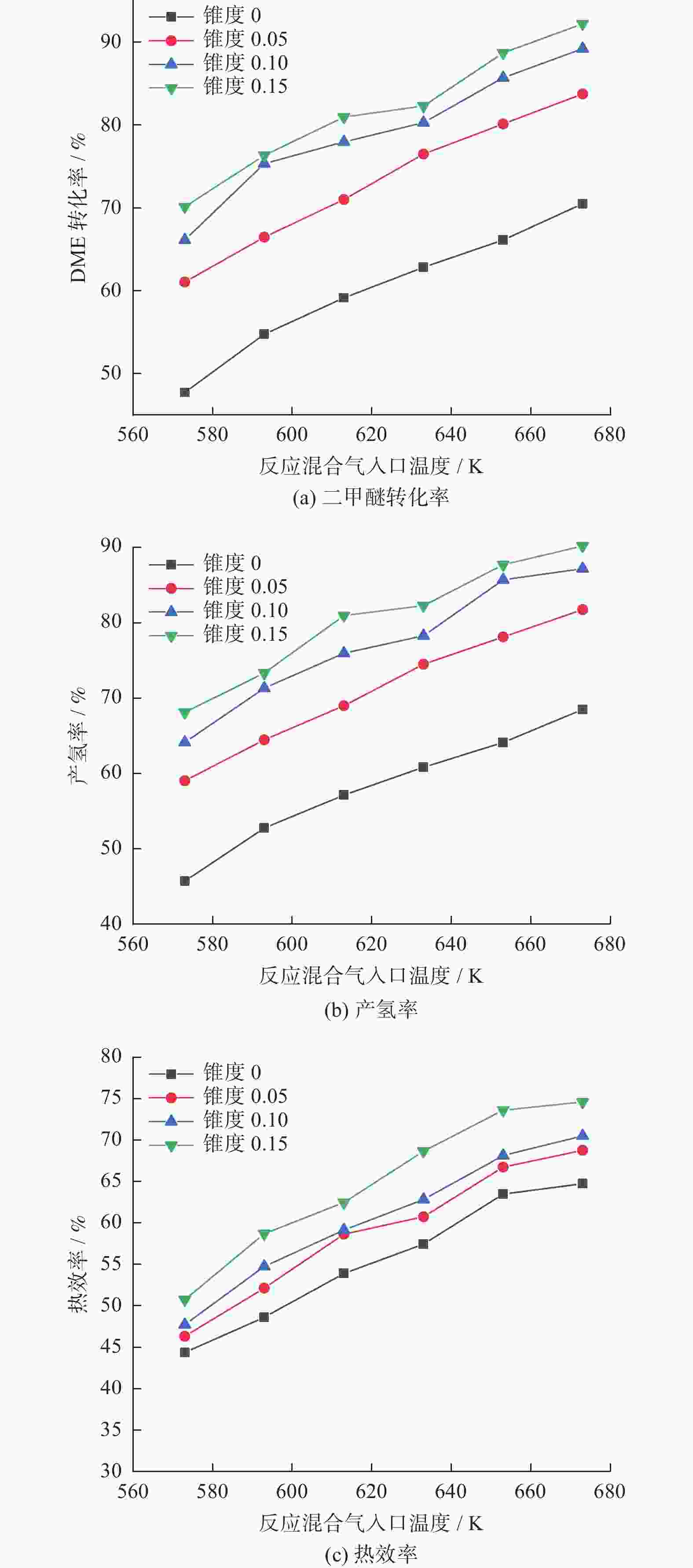
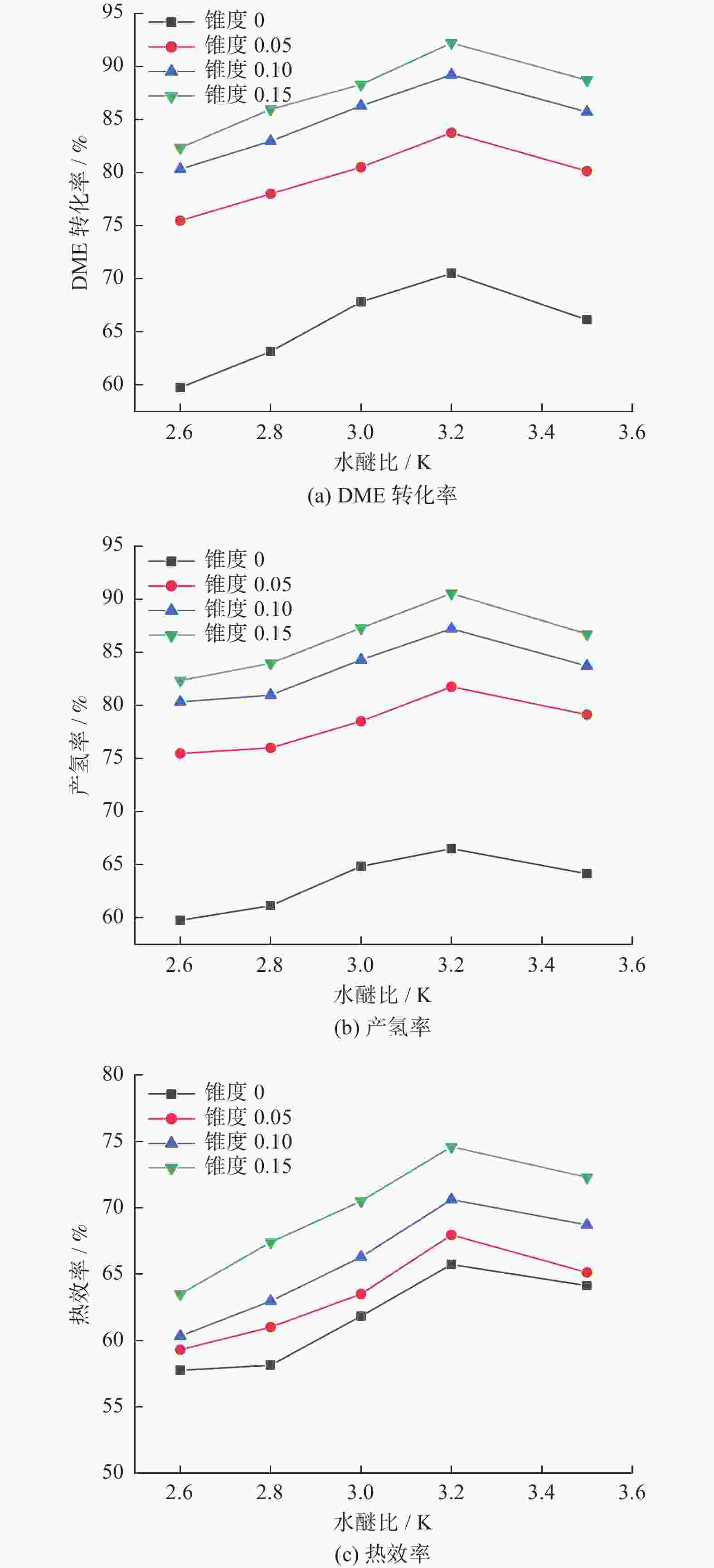
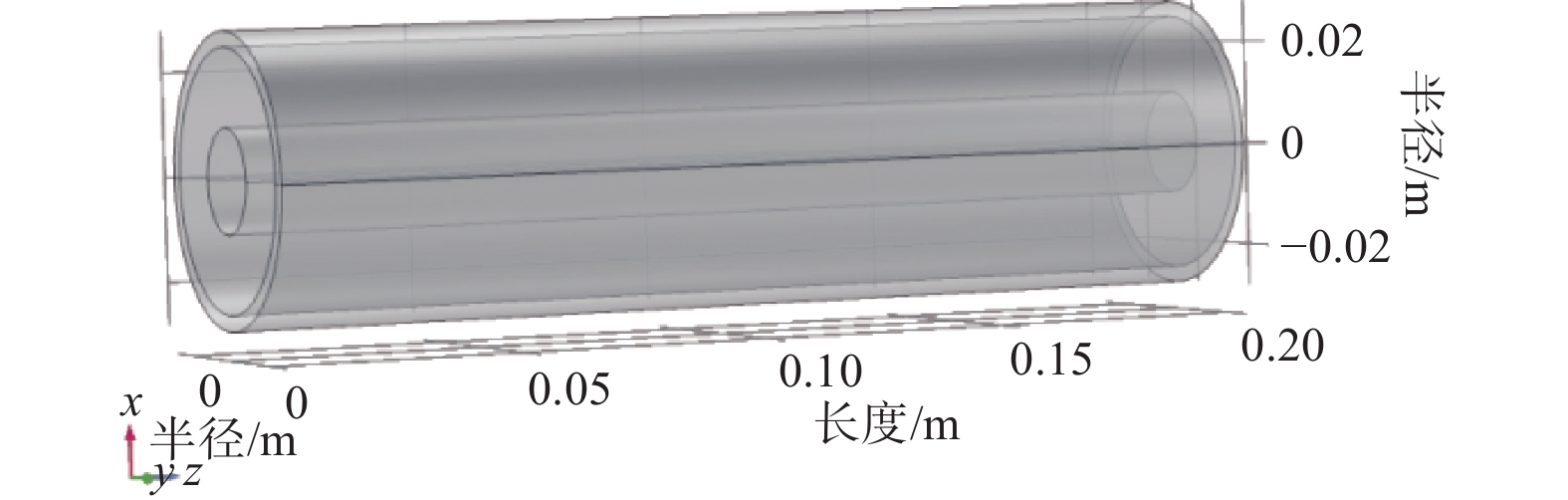
摘要: 设计了一种圆台形二甲醚水蒸气重整制氢反应器,并建立了二甲醚水蒸气重整制氢反应系统数值模型,利用COMSOL软件对建立的数值模型进行求解,仿真和实验的数值结果基本一致. 通过对重整反应器的结构进行优化,获得更高的二甲醚转化率,研究圆台锥度变化对重整反应的影响,分析反应条件对二甲醚转化和制氢的影响. 结果表明,在一定范围内增加锥度时,可以获得较高的产氢率和热效率. 通过结构优化,二甲醚水蒸气重整反应系统可获得92.21%的二甲醚转化率,90.54%的产氢率,热效率最高可达74.6%.Abstract: A frustum of a cone for dimethyl ether (DME) steam reforming hydrogen production was designed, and the numerical model of DME steam reforming hydrogen production reaction system was established. The numerical model was solved by COMSOL software, the numerical results of simulation and experiment were basically consistent. By optimizing the structure of the reforming reactor, higher DME conversion rate was obtained. The influence of the cone change on the reforming reaction , and the influence of reaction conditions on DME conversion and hydrogen production were analyzed. The results showed that higher hydrogen yield and thermal efficiency can be obtained When increasing the taper within a certain range. Through structural optimization, the DME steam reforming reaction system can obtain 92.21% DME conversion and 90.54% hydrogen production rate. The highest thermal efficiency is 74.6%.
-
Key words:
- hydrogen reforming /
- numerical model /
- structure optimization /
- frustum of a cone
-
表 1 DME 重整反应的动力学模型及反应速率
Table 1. Kinetic model and reaction rate of DME reforming reaction
反应名称 化学方程式 反应速率 二甲醚水解 $\mathrm{CH}_3 \mathrm{OCH}_3+\mathrm{H}_2 \mathrm{O}=2 \mathrm{CH}_3 \mathrm{OH}$ $\left( {1 - \varepsilon } \right)\rho {\text{•} } \exp\left( { - {E_{\rm{H}}}/RT} \right){C_{{\rm{DME}}} }$[16] 甲醇水蒸气重整反应 $\mathrm{CH}_3 \mathrm{OH}+\mathrm{H}_2 \mathrm{O} \Leftrightarrow \mathrm{CO}_2+3 \mathrm{H}_2$ $\begin{gathered} \left( {1 - \varepsilon } \right)\rho {\text{•} } {k_R}. {\rm{exp} }\left( { - {E_{\rm{H}}}/RT} \right){C_{{\rm{CH}}_3{\rm{OH}}} } \\ {k_R} = 5.5•(1.15\text{•}1{0^6} + 9.41\text{•}1{0^5}\text{•}\log\Phi ) {\rm{exp} }\left( { - {E_R}/RT} \right) \\ \end{gathered}$[16] 水煤气变换反应 $\mathrm{CO}+\mathrm{H}_2 \mathrm{O} \Leftrightarrow \mathrm{CO}_2+\mathrm{H}_2$ $\begin{array}{*{20}{l} } {11.2 {k_{{\rm{WGS}}} }\left( { {P_{{\rm{CO}}} }{P_{{\rm{H}}_2{\rm{O}}} } - {P_{{\rm{co}}_2} }{P_{{\rm{H}}_2} }/{k_{{\rm{eq}}} } } \right)} \\ { {K_{{\rm{WGS}}} } = 1.74\text{•}1{0^{17} }(1 - 0.154\;0\delta + 0.008{\delta ^2}){T^{ - 8.5} }{\rm{exp} }\left( { - 3\;500/(RT)} \right)} \\ {k_{{\rm{eq}}} } = {\rm{exp} }\left( {4\;577.8/T - 4.33} \right) \end{array}$[16] -
[1] ADENIYI A G, IGHALO J O, ELETTA O A A. Process integration and feedstock optimisation of a two-step biodiesel production process from jatropha curcas using aspen plus[J] . Chemical Product and Process Modeling,2018,14(2):2018 − 0055. [2] FAN F Y, ZHAO L, HOU H, ZHANG Q. Insights into the CO formation mechanism during steam reforming of dimethyl ether over NiO/Cu-based catalyst[J] . Industrial & Engineering Chemistry Research,2019,58(8):3440 − 3449. [3] 刘江华. 氢能源: 未来的绿色能源[J] . 现代化工,2006(S2):10 − 13,15. doi: 10.16606/j.cnki.issn0253-4320.2006.s2.003 [4] KAJORNSAK F, NAWIN V, WIWUT T. Evaluation of the thermodynamic equilibrium of the autothermal reforming of dimethyl ether[J] . International Journal of Hydrogen Energy,2011,36(10):5865 − 5874. doi: 10.1016/j.ijhydene.2011.02.027 [5] SONG J W, CHOI M Y, LEE J, et al. Improvement of fuel economy and greenhouse gases reduction in gasoline powered vehicles through the TWC-NOx trap catalyst[J] . International Journal of Automotive Technology,2020,21(2):441 − 449. doi: 10.1007/s12239-020-0041-8 [6] SWMWISBERGER T A, BORUP R L. Thermodynamic equilibrium calculations of dimethyl ether steam reforming and dimethyl ether hydrolysis[J] . Journal of Power Soures,2005,152(10):87 − 96. [7] FENG D M, WANG Y Y, WANG D, et al. Steam reforming of dimethyl ether over Cu-ZnO-Al2O3-ZrO2 + ZSM-5: A kinetic study[J] . Chemical Engineering Journal,2009,146(2):477 − 485. [8] WANG S, ISHIHARA T, TAKITA Y. Partial oxidation of dimethyl ether over various supported metal catalysts[J] . Applied Catalysis A: General,2002,228(1/2):167 − 176. doi: 10.1016/S0926-860X(01)00985-1 [9] LI C, GAO Y, WU C. Modeling and simulation of hydrogen production from dimethyl ether steam reforming using exhaust gas[J] . International Journal of Energy Research,2015,39(9):1272 − 1279. doi: 10.1002/er.3330 [10] 寇素原, 王晓蕾, 任克威, 等. 二甲醚水蒸气重整制氢过程的热力学分析[J] . 天然气化工(C1化学与化工),2009,34(1):35 − 40. [11] CHEN Y, ZHANG C, WU R, et al. Methanol steam reforming in microreactor with constructal tree-shaped network[J] . Journal of Power Sources,2011,196(15):6366 − 6373. doi: 10.1016/j.jpowsour.2011.03.044 [12] YAO F, CHEN Y, PETERSON G P. Hydrogen production by methanol steam reforming in a disc microreactor with tree-shaped flow architectures[J] . International Journal of Heat and Mass Transfer,2013,64:418 − 425. doi: 10.1016/j.ijheatmasstransfer.2013.04.057 [13] AN H, LI A, SASMITO A P, et al. Computational fluid dynamics (CFD) analysis of micro-reactor performance: Effect of various configurations[J] . Chemical Engineering Science,2012,75:85 − 95. doi: 10.1016/j.ces.2012.03.004 [14] CHEN S Y, Li C, REN H J. Design and optimization of reforming hydrogen production reaction system for automobile fuel cell[J] . International Journal of Hydrogen Energy,2021,46(49):25252 − 25263. doi: 10.1016/j.ijhydene.2021.05.035 [15] XU D, LI C. Design and optimization of dimethyl ether steam-reforming reactor[J] . Journal of Energy Engineering,2022,148(2):1943. [16] ZHANG T Q, OU K, JUNG S H, et al. Dynamic analysis of a PEM fuel cell hybrid system with an on-board dimethyl ether (DME) steam reformer (SR)[J] . International Journal of Hydrogen Energy,2018,43(29):13521 − 13531. doi: 10.1016/j.ijhydene.2018.05.098 -





 下载:
下载: