Thermodynamic analysis of methane dry reforming for H2/CO syngas production
-
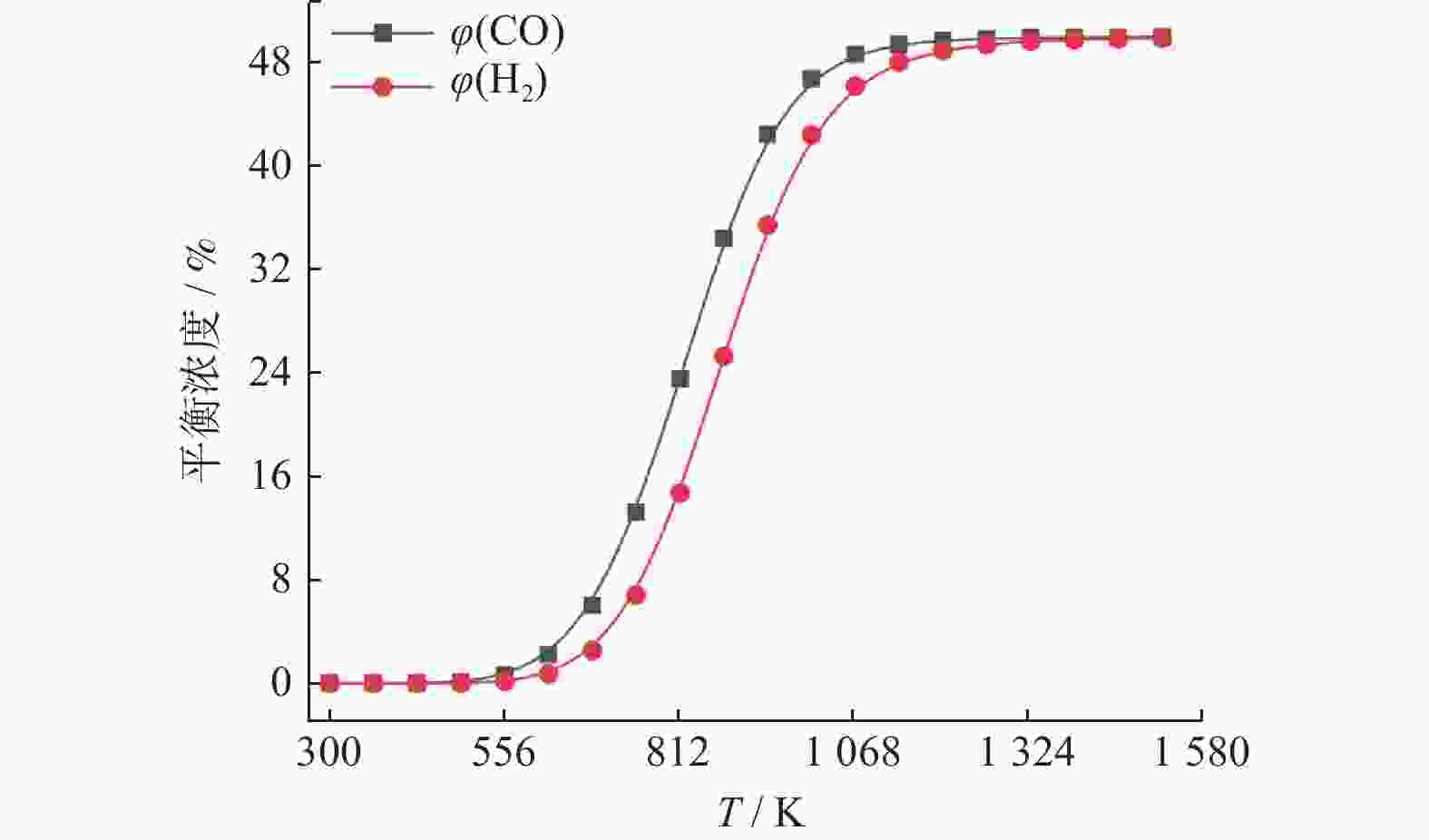
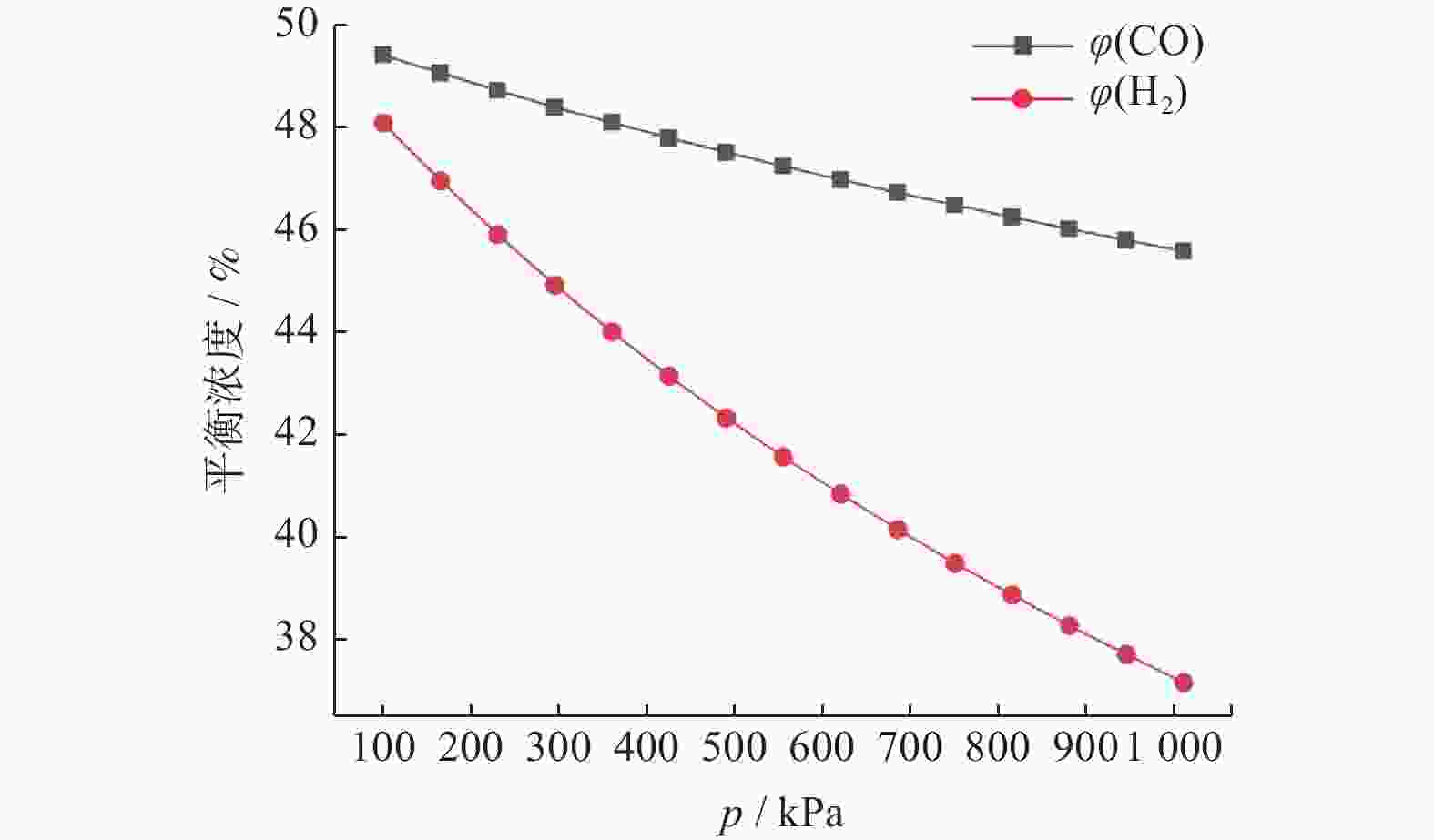
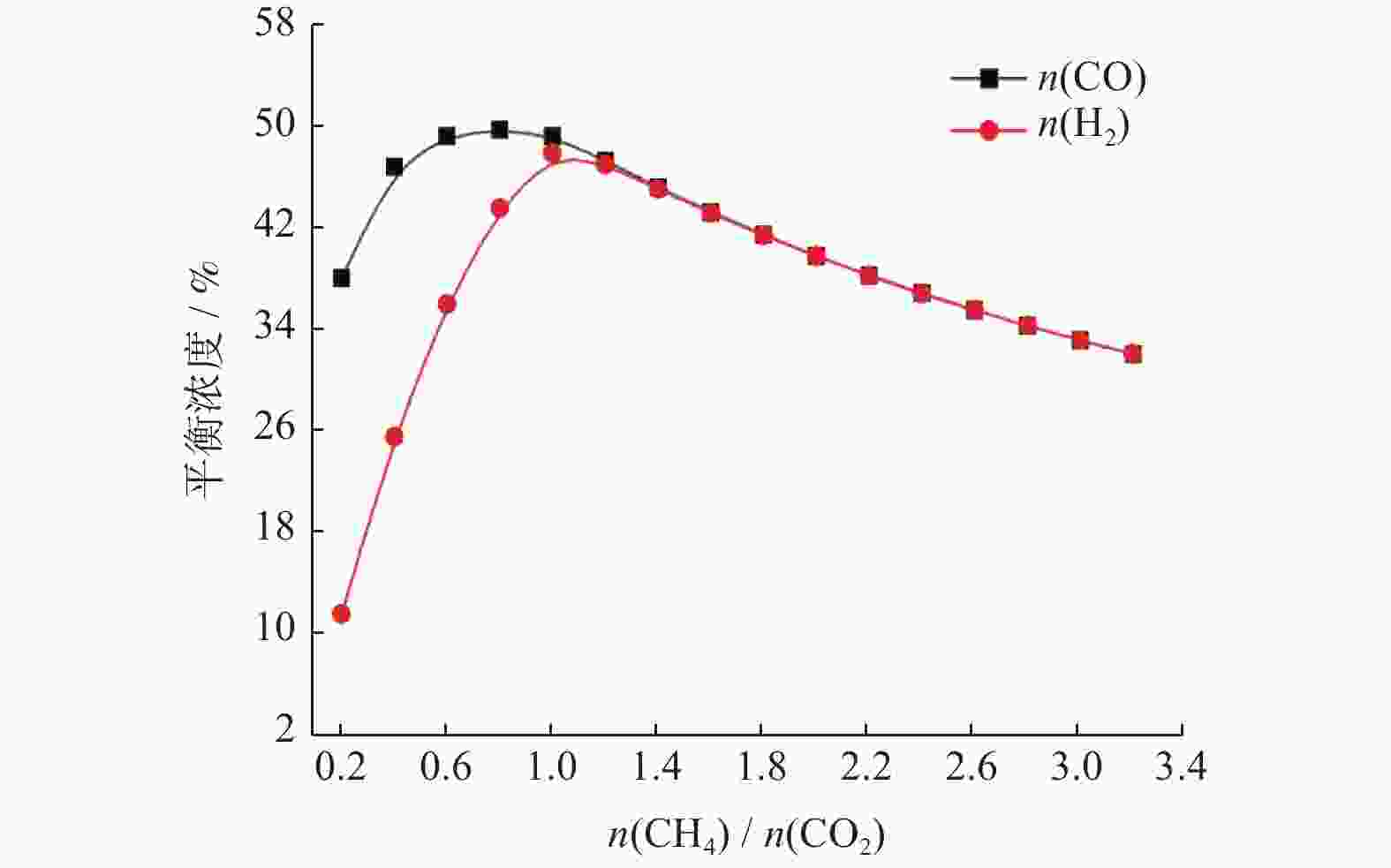
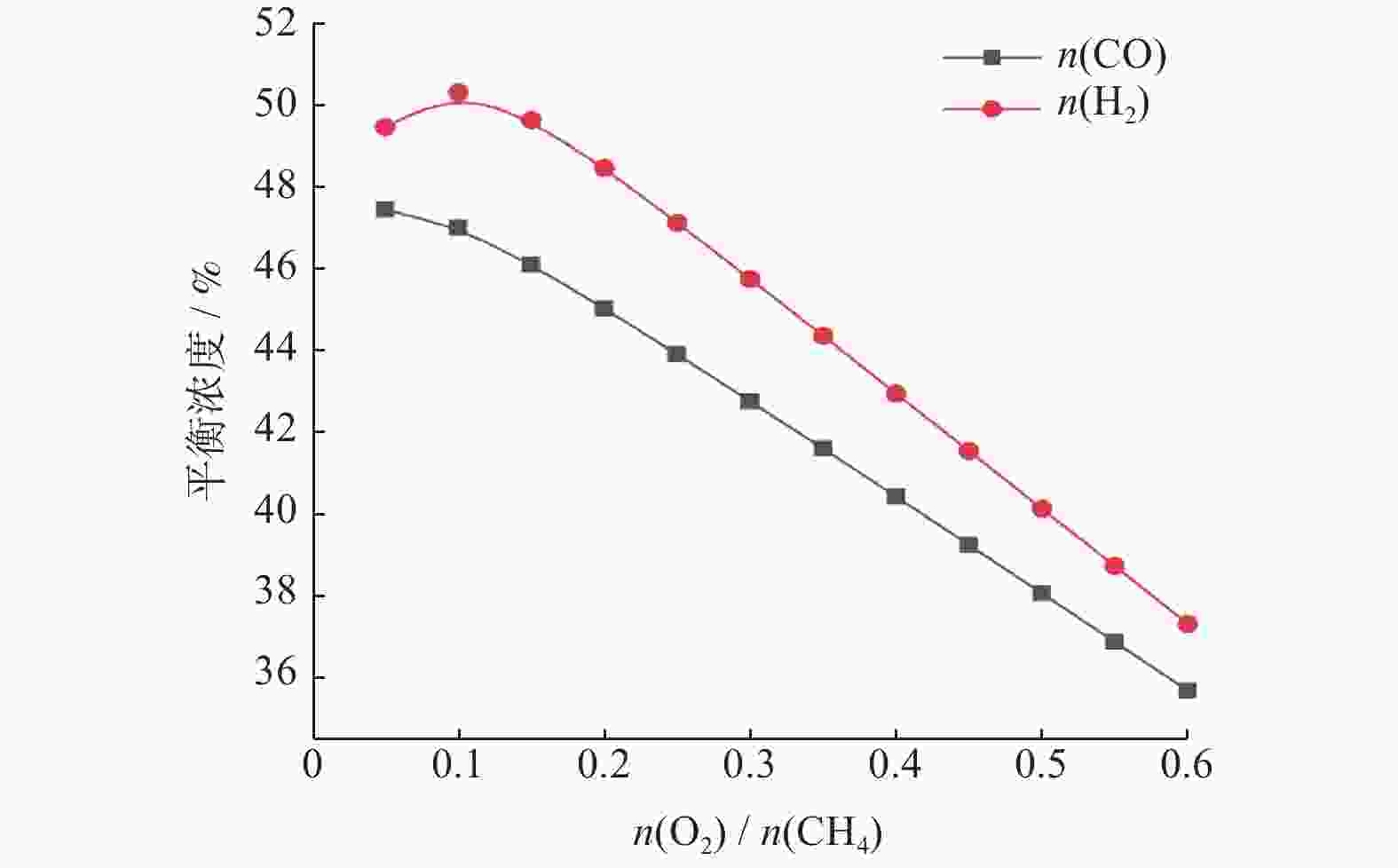
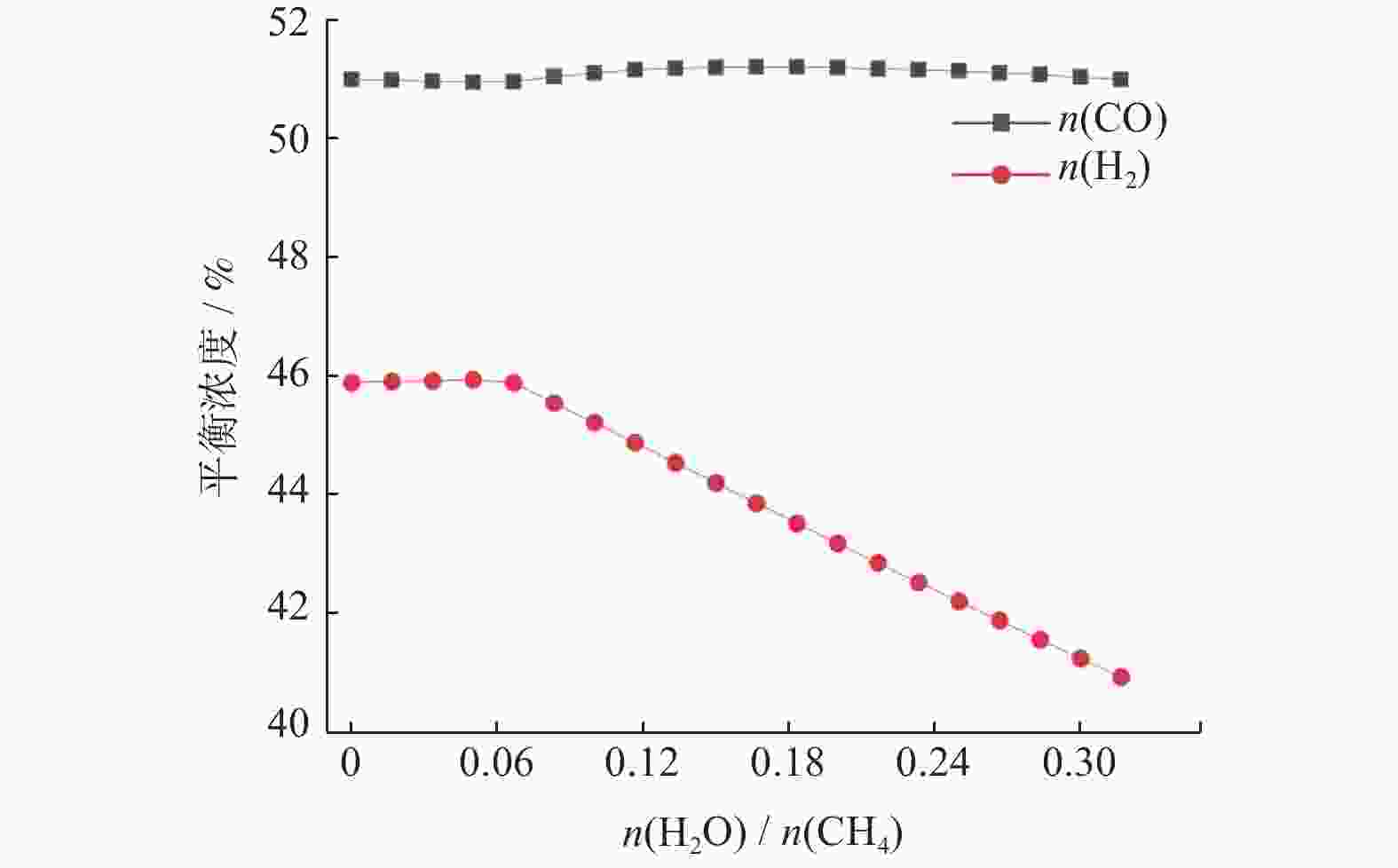
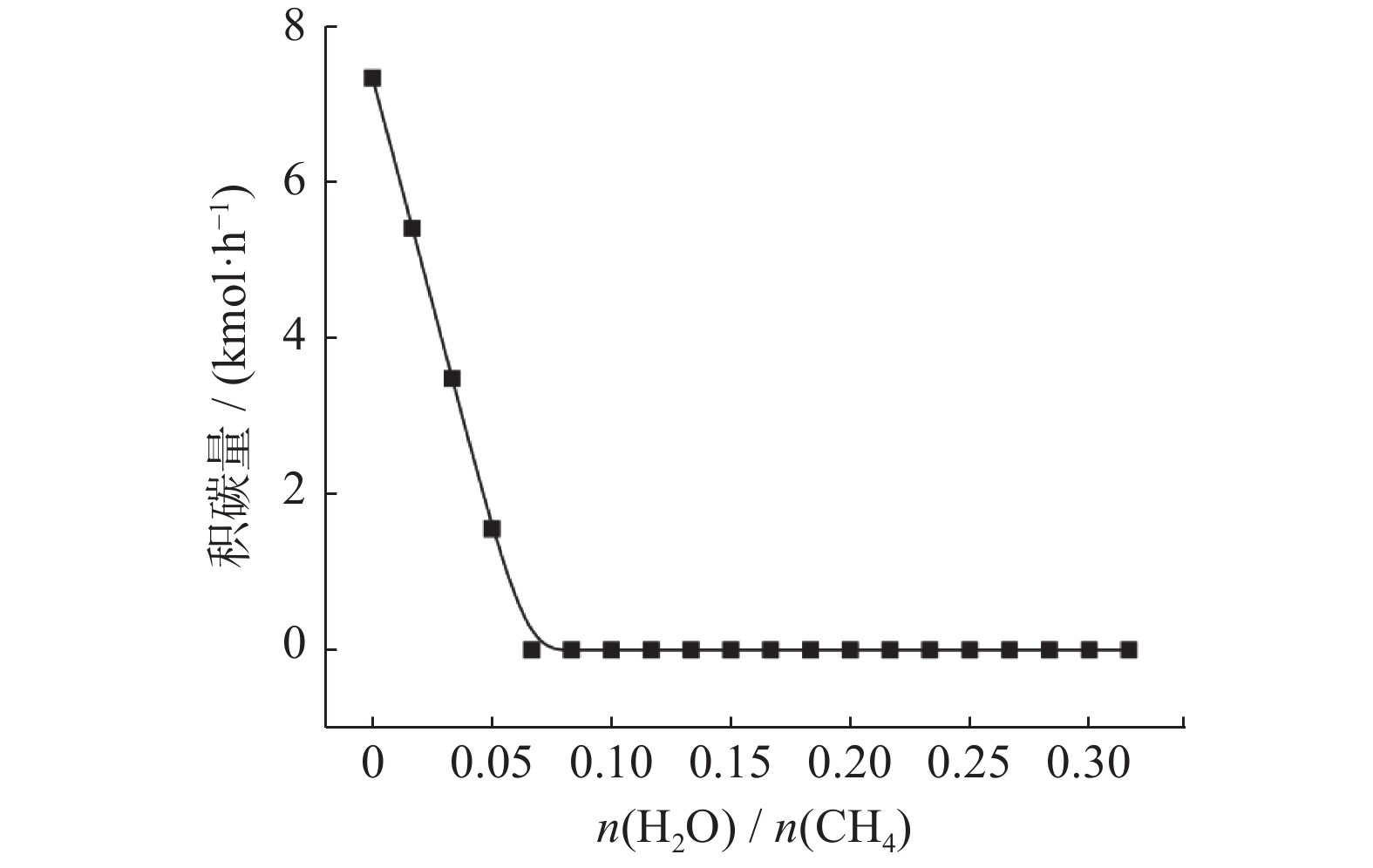
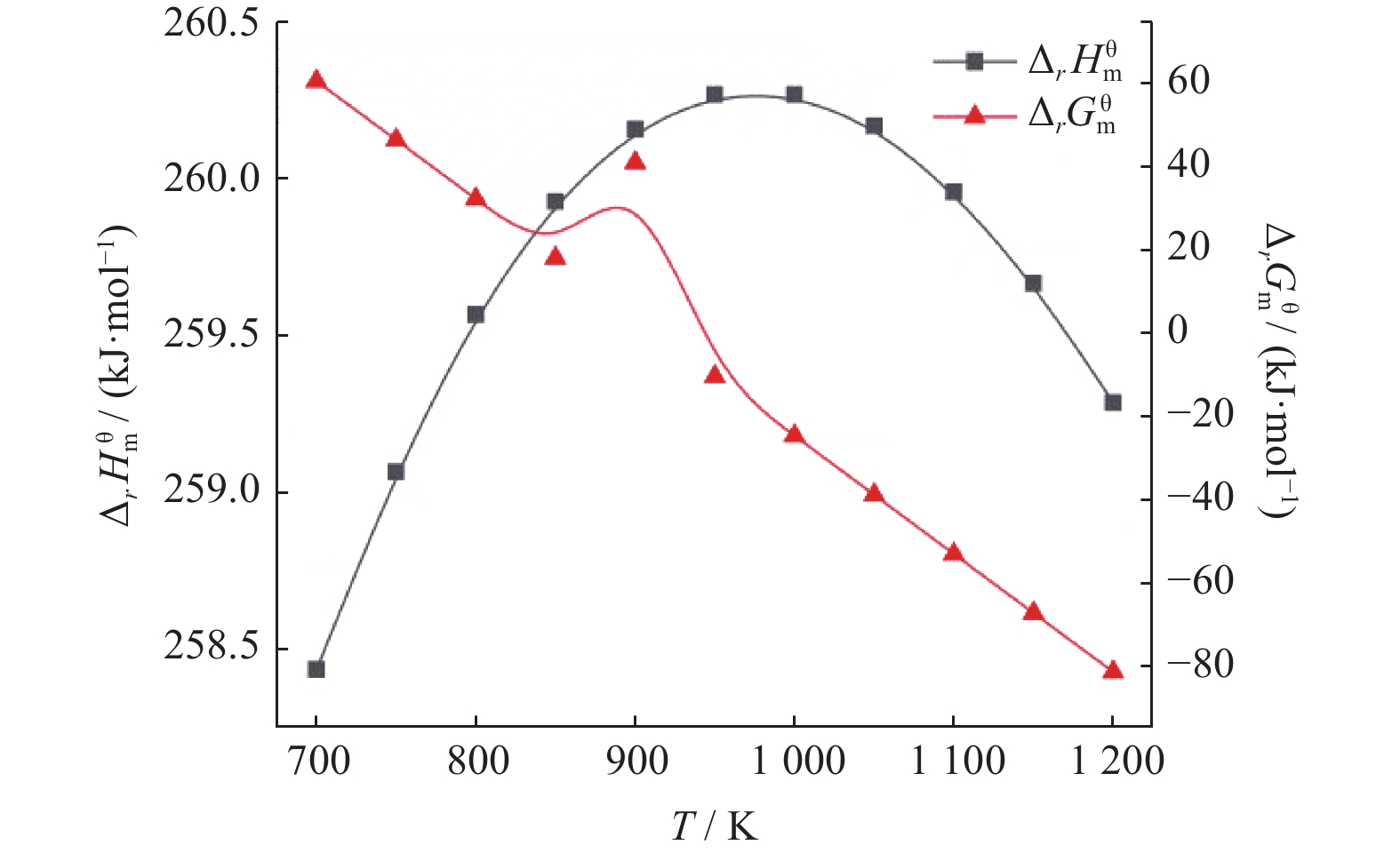
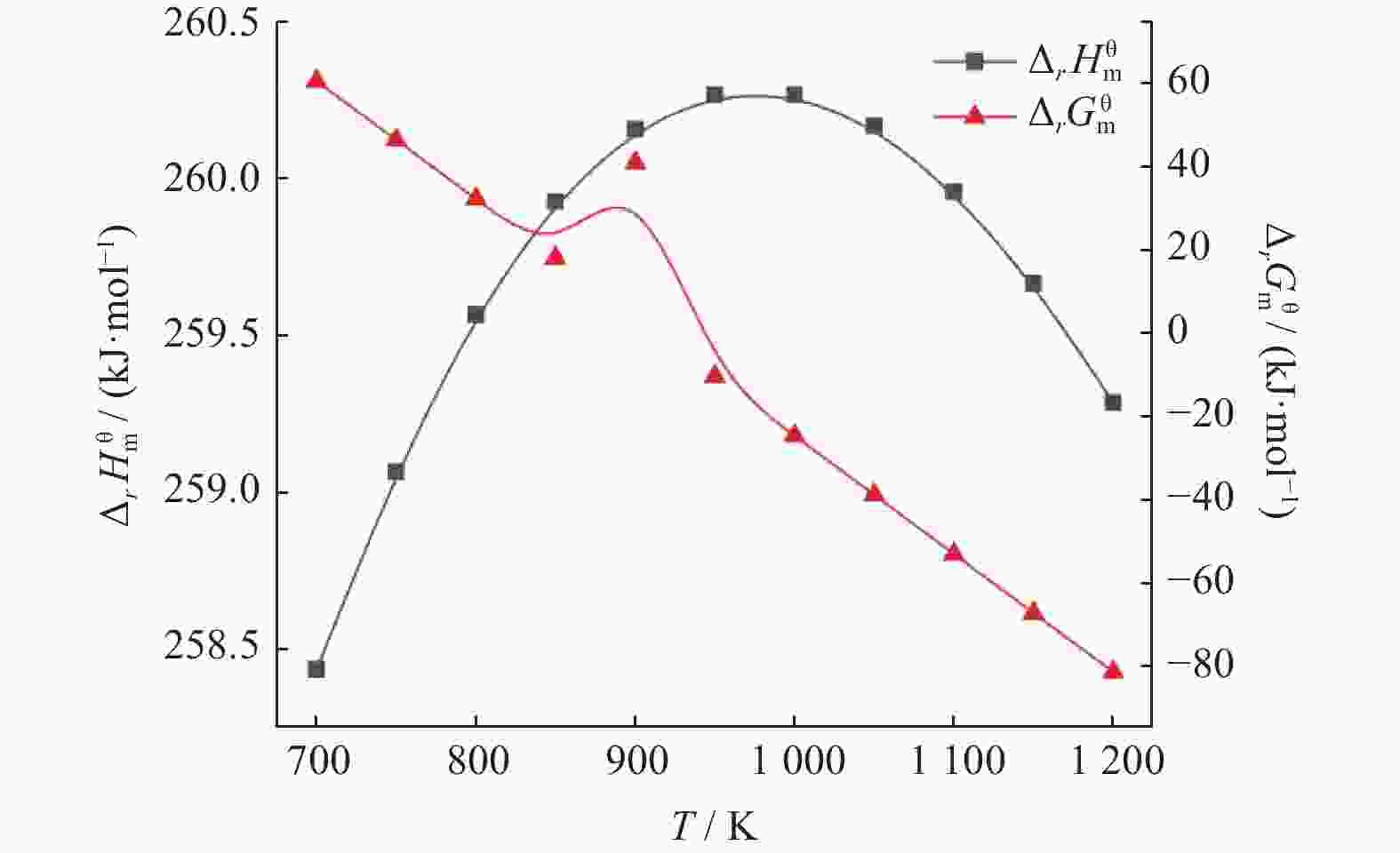
摘要: 基于吉布斯自由能最小化原理,采用HSC Chemistry软件对甲烷干重整反应过程进行热力学分析,系统探讨反应温度、压力、进料比(物质的量比n(CH4)/n(CO2))、O2进量和H2O进量等关键参数对CO和H2平衡浓度、n(H2)/n(CO)及积碳量的影响。研究表明,当温度处于
1137 K,压力为101.325 kPa,n(CH4)/n(CO2)为1.20,n(O2)/n(CH4)为0.15和n(H2O)/n(CH4)为0.07时,n(H2)/n(CO)保持在0.90左右,接近理论值1。甲烷干重整可以同时实现温室气体的减排及高值化利用,制备的合成气是燃料和重要的化工原料气,是实现“双碳”目标的前景技术之一。Abstract: The thermodynamic analysis of the methane dry reforming process was conducted based on Gibbs free energy minimization principle using HSC Chemistry software. The effects of key parameters including reaction temperature, pressure, feed ratio (n(CH4)/n(CO2) mole ratio), O2 and H2O feed rates on equilibrium concentration of CO and H2, n(H2)/n(CO) mole ratio and carbon deposition were systematically investigated. It is shows that under conditions of1137 K reaction temperature, 101.325 kPa pressure, n(CH4)/n(CO2) ratio of 1.20, n(O2)/n(CH4) ratio of 0.15, and n(H2O)/n(CH4) ratio of 0.07, the n(H2)/n(CO) ratio is maintained at approximately 0.90, closely approaching the theoretical value of 1. The methane dry reforming enables both greenhouse gas reduction and high-value utilization, with the produced syngas serving as fuel and important chemical feed gas, making it a promising technology for achieving dual carbon goals.-
Key words:
- methane /
- dry reforming /
- H2/CO syngas. /
- thermodynamics /
- equilibrium
-
表 1 CH4和CO2转化过程中的相关反应
Table 1. Related reactions during CH4 and CO2 conversion process
序号 1 2 3 4 5 6 7 8 反应物 CH4 + CO2 CO2 + H2 CO2 + H2 CH4 + CO2 CO CH4 CO + H2 CH4 + O2 生成物 CO + H2 CO + H2O CH4 + H2O CO + H2O C + CO2 C + 2H2 C + H2O CO2 + H2O -
[1] 刘勇跃. 基于ASPEN模拟计算的甲烷二氧化碳重整反应研究[J] . 化工设计,2018,28(4):16 − 21. doi: 10.3969/j.issn.1007-6247.2018.04.006 [2] GUO J Z, HOU Z Y, ZHENG X M. Autothermal reforming of CH4 and C3H8 to syngas in a fluidized-bed reactor[J] . Chinses Journal of Catalysis,2010,31(9):1115 − 1121. [3] YUSUF M, BEG M, UBAIDULLAH M, et al. Kinetic studies for DRM over high-performance Ni–W/Al2O3–MgO catalyst[J] . International Journal of Hydrogen Energy,2022,47(100):42150 − 42159. doi: 10.1016/j.ijhydene.2021.08.021 [4] 刘俊义, 祝贺, 张军. 甲烷二氧化碳自热重整工艺分析[J] . 天然气化工(C1化学与化工),2019,44(3):56 − 60. [5] DEMIDOV D V, MISHIN I V, MIKHAILOV M N. Gibbs free energy minimization as a way to optimize the combined steam and carbon dioxide reforming of methane[J] . International Journal of Hydrogen Energy,2011,36(10):5941 − 5950. [6] 赵倩, 丁干红. 甲烷二氧化碳重整工艺研究及经济性分析[J] . 天然气化工(C1化学与化工),2020,45(4):71 − 75, 81. [7] ERDOHELI A, CSSERENYI J, SOLYMOSI F. Activation of CH4 and its reaction with CO2 over supported Rh catalysts[J] . Catalysis,1993,141(1):287 − 299. [8] 吕长剑, 王娟. 二氧化碳甲烷化技术进展与应用分析[J] . 炼油技术与工程,2024,54(4):6 − 10. [9] 石天宝, 张秋菊, 纪容昕, 等. CH4和CO2转化催化剂的研究[J] . 化工生产与技术,2000,7(2):12 − 15. doi: 10.3969/j.issn.1006-6829.2000.02.003 [10] 傅献彩, 沈文霞, 姚天扬, 等. 物理化学(第五版)[M] . 北京: 高等教育出版社, 2009. [11] 张迪茜. 生物质能源研究进展及应用前景[D] . 北京: 北京理工大学, 2015. [12] 李建伟, 陈冲, 王丹, 等. 甲烷二氧化碳重整热力学分析[J] . 石油与天然气化工,2015,44(3):60 − 64. doi: 10.3969/j.issn.1007-3426.2015.03.013 [13] JENSEN C, DUYAR M S. Thermodynamic analysis of dry reforming of methane for valorization of landfill gas and natural gas[J] . Energy Technology,2021,9(7):2100106. [14] USMAN M, Wan DAUD W M A, ABBAS H F. Dry reforming of methane: influence of process parameters: a review[J] . Renewable and Sustainable Energy Reviews,2015,45:710 − 744. doi: 10.1016/j.rser.2015.02.026 [15] 井德水, 方志刚, 张树友, 等. 氧气/甲烷比对Mo/Al2O3催化剂上甲烷二氧化碳重整反应的影响[J] . 天然气化工(C1化学与化工),2016,41(6):54 − 58. [16] 郭禹. 甲烷二氧化碳重整制合成气镍基催化剂的设计制备[D] . 北京: 北京化工大学, 2022. -





 下载:
下载: