Optimization of preparation process of clove volatile oil inclusion complex by Box-Behnken response surface methodology
-
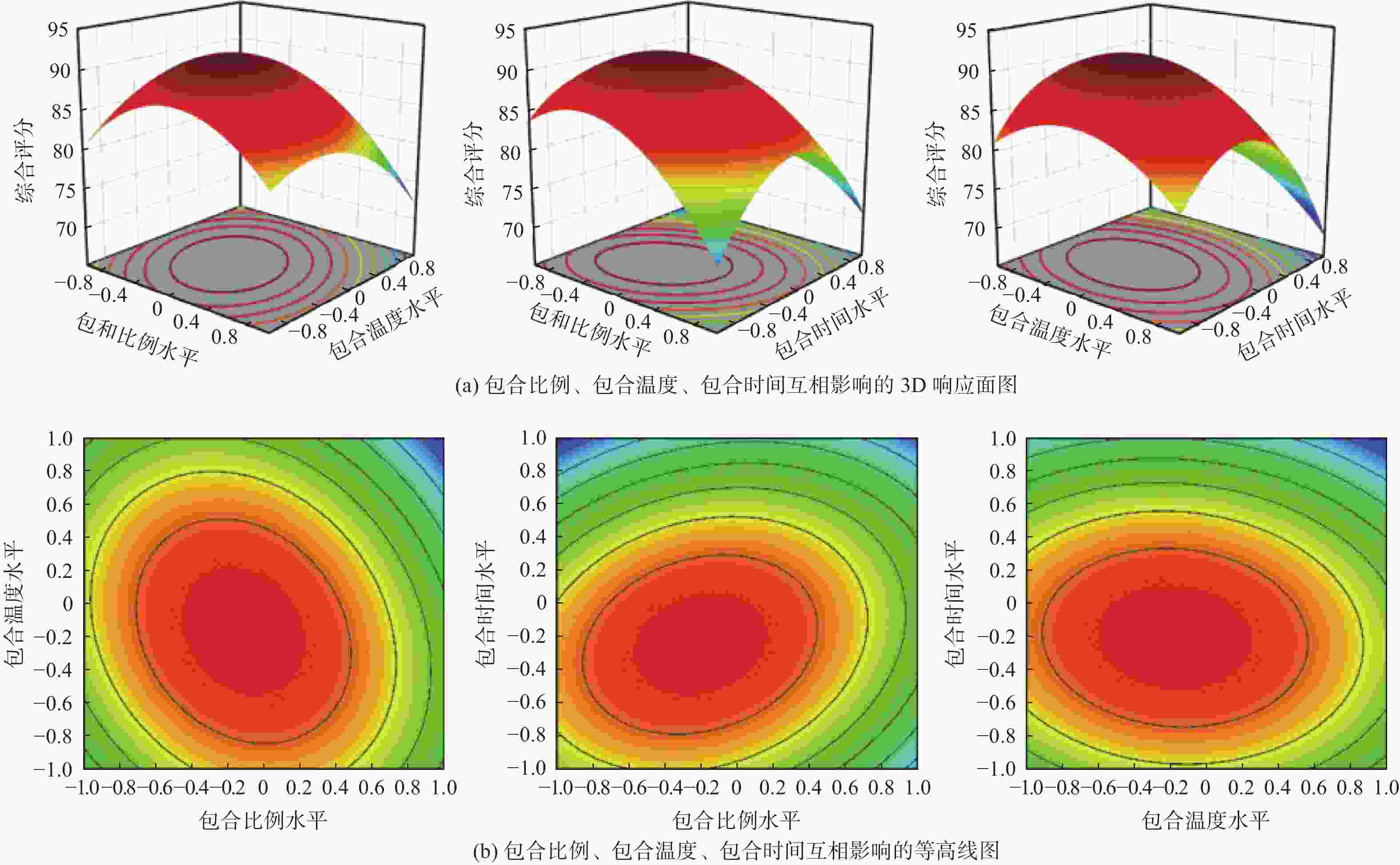
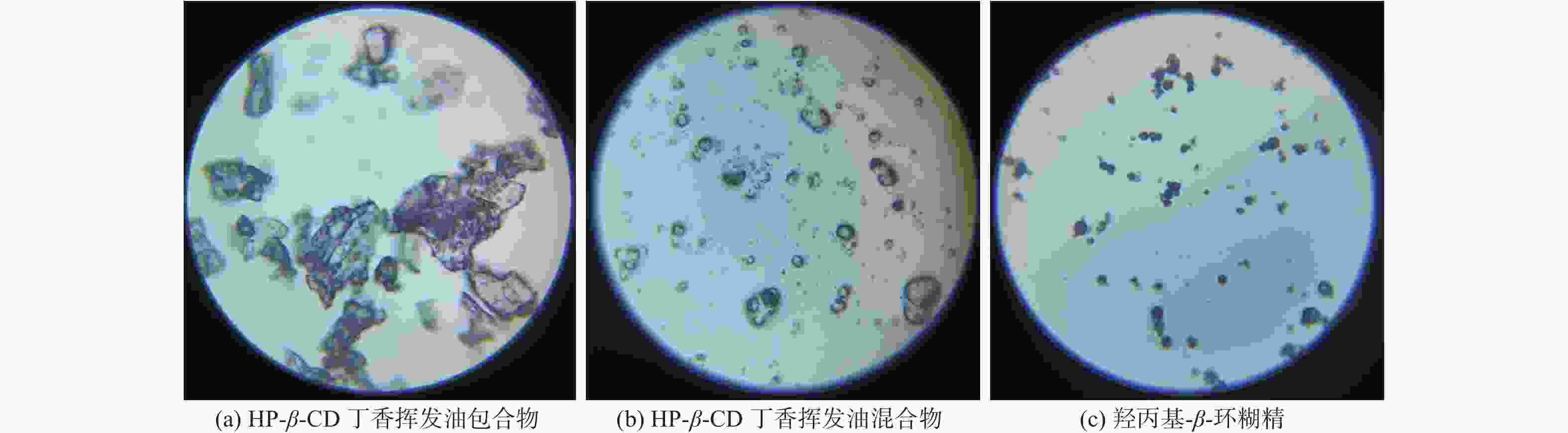
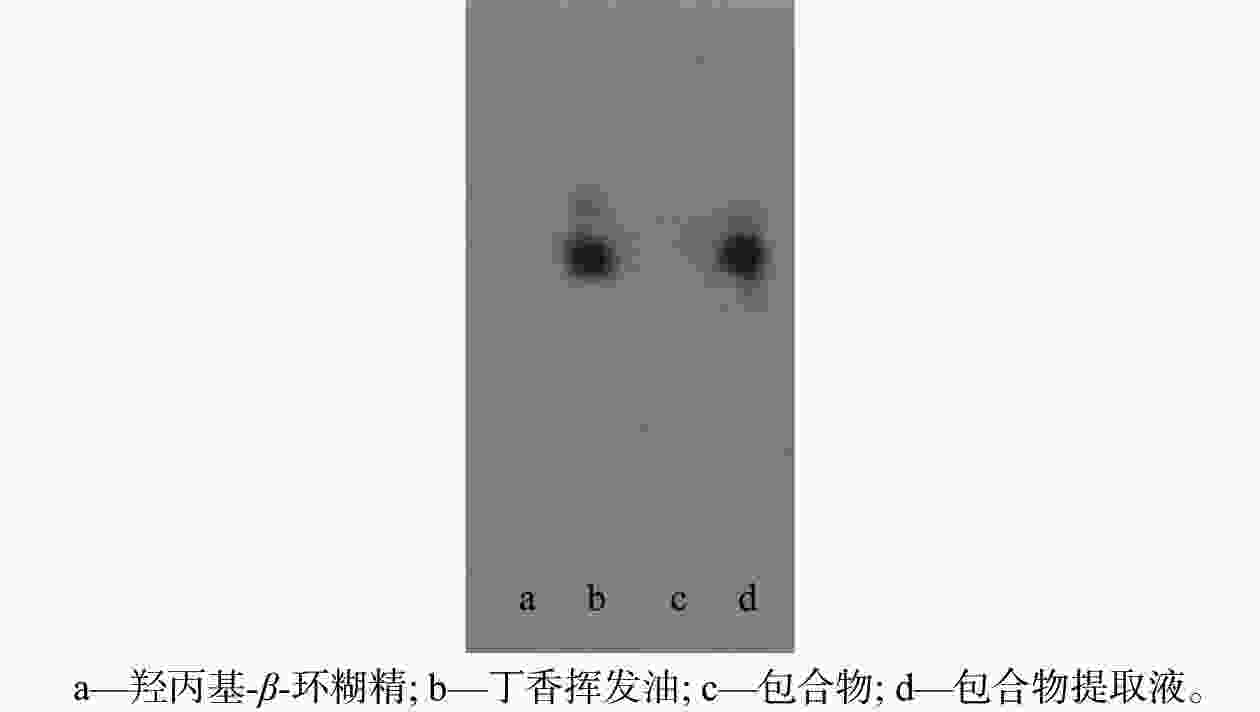
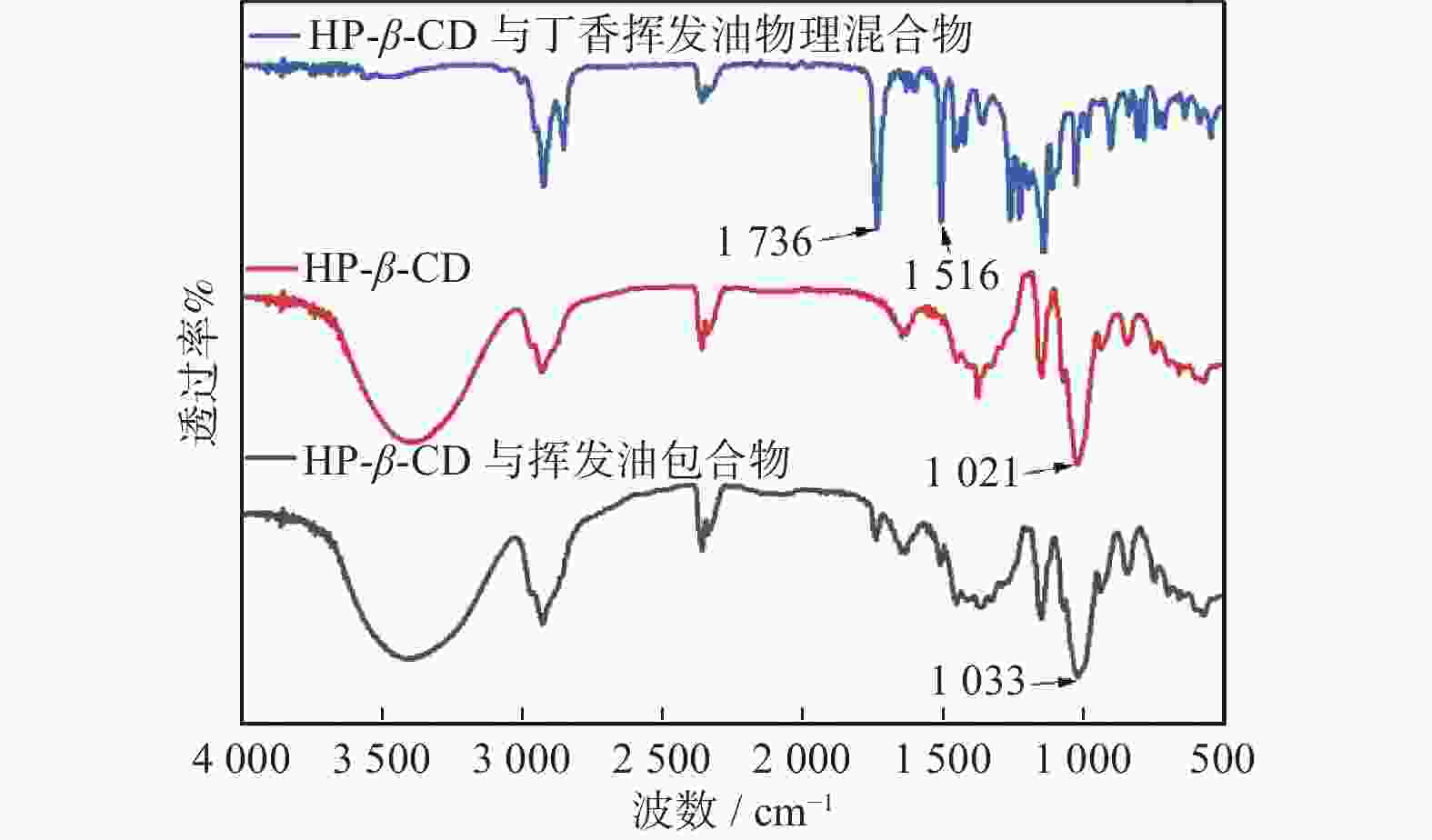
摘要: 采用饱和水溶液法制备丁香挥发油羟丙基-β-环糊精包合物。在单因素实验的基础上,选择羟丙基-β-环糊精与挥发油比例、包合温度、包合时间作为自变量,以综合评分为响应值,采用Box-Behnken响应面法优化包合工艺,得到最佳包合工艺为:羟丙基-β-环糊精与挥发油的混合比例为14.5∶1,包合温度59 ℃,包合时间2.4 h。3组验证实验的综合评分为92.21%±0.50%,与预测值接近,表明该模型的预测准确可靠。该包合物经显微镜观察,薄层色谱法(TLC)分析,红外光谱鉴别,证明已形成稳定的包合物,为进一步开发利用丁香挥发油提供数据支持。
-
关键词:
- 丁香挥发油 /
- 羟丙基-β-环糊精 /
- 工艺优化 /
- Box-Behnken响应面法
Abstract: To prepare the hydroxypropyl-β-cyclodextrin inclusion complex from clove essential oil using saturated aqueous solution method. Based on the single factor experiments, the ratio of hydroxypropyl-β-cyclodextrin to volatile oil, encapsulation temperature, and encapsulation time were used as influencing factors, and the comprehensive score was used as the response value. The encapsulation process was optimized by Box-Behnken response surface methodology. The optimal condition was obtained as the ratio of hydroxypropyl-β-cyclodextrin to volatile oil was 14.5∶1, with an inclusion temperature of 59 ℃ and an inclusion time of 2.4 h. The comprehensive score of the three validation experiments is 92.21%±0.50%, which is close to the predicted value, indicating that the prediction of the model is accurate and reliable. The inclusion complex was observed under microscope, analyzed by thin layer chromatography (TLC), and identified by infrared spectroscopy, proving the formation of a stable inclusion complex. These results can provide data support for further development and utilization of clove volatile oil. -
表 1 因素水平表
Table 1. Factor levels table
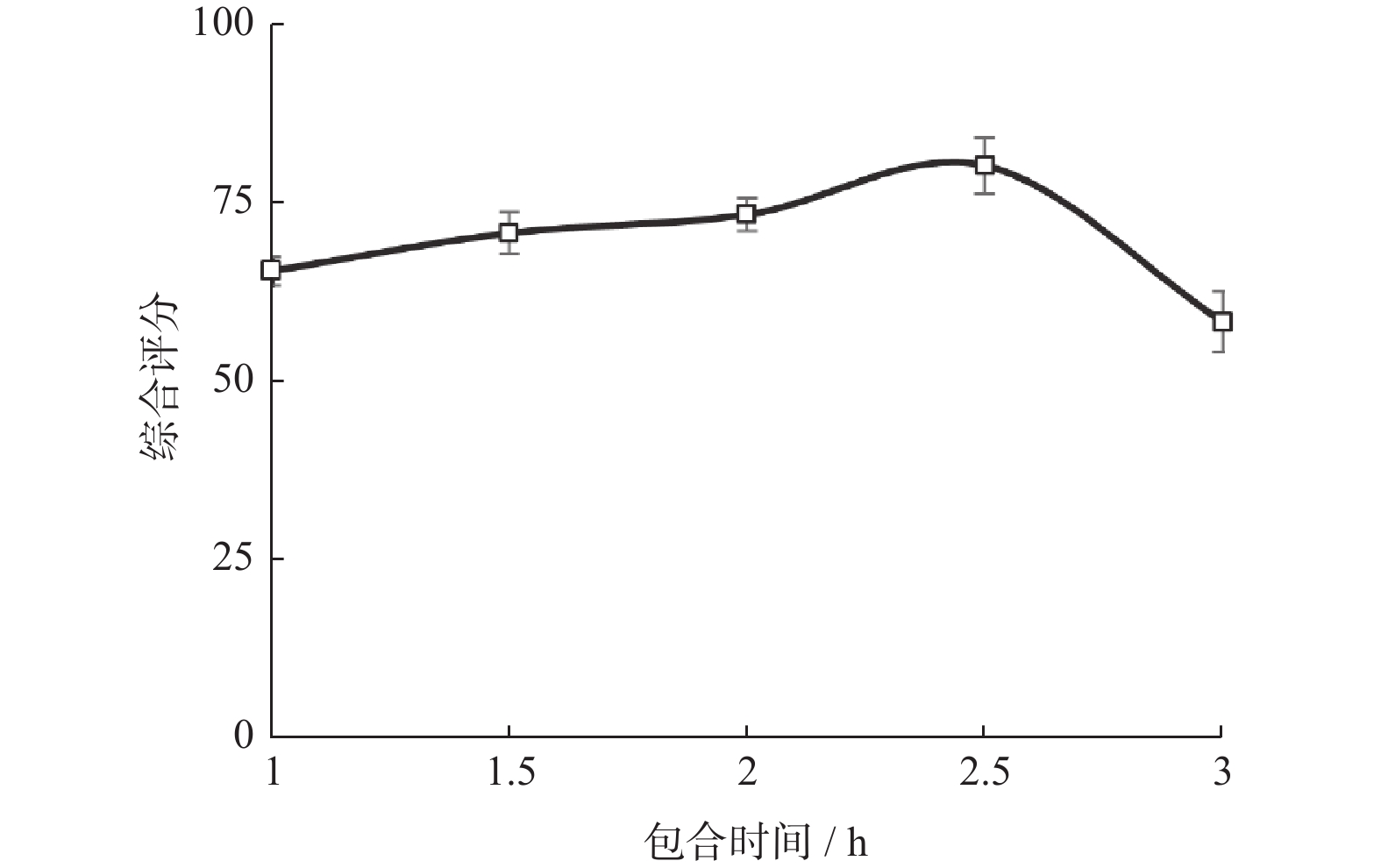
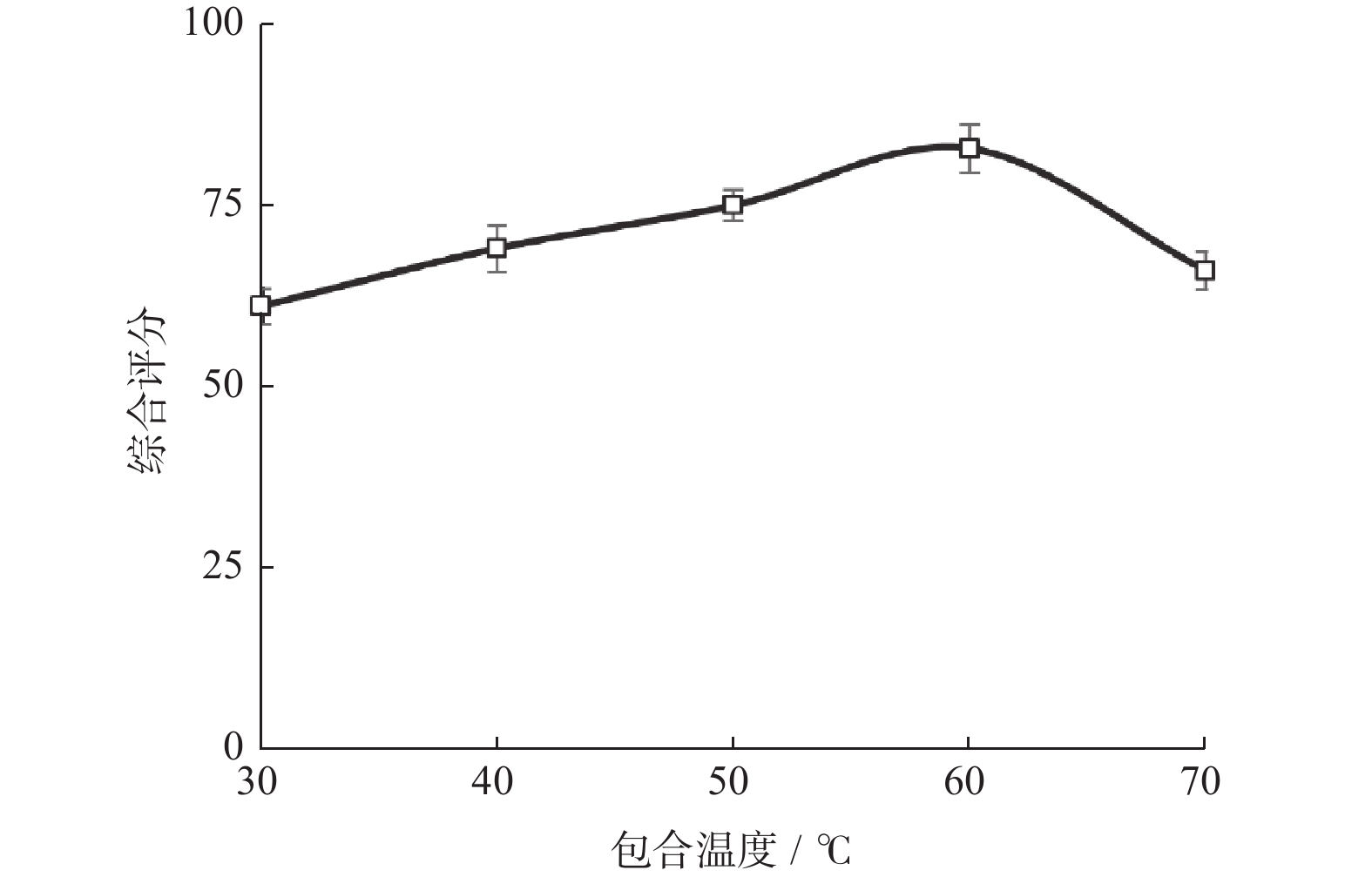
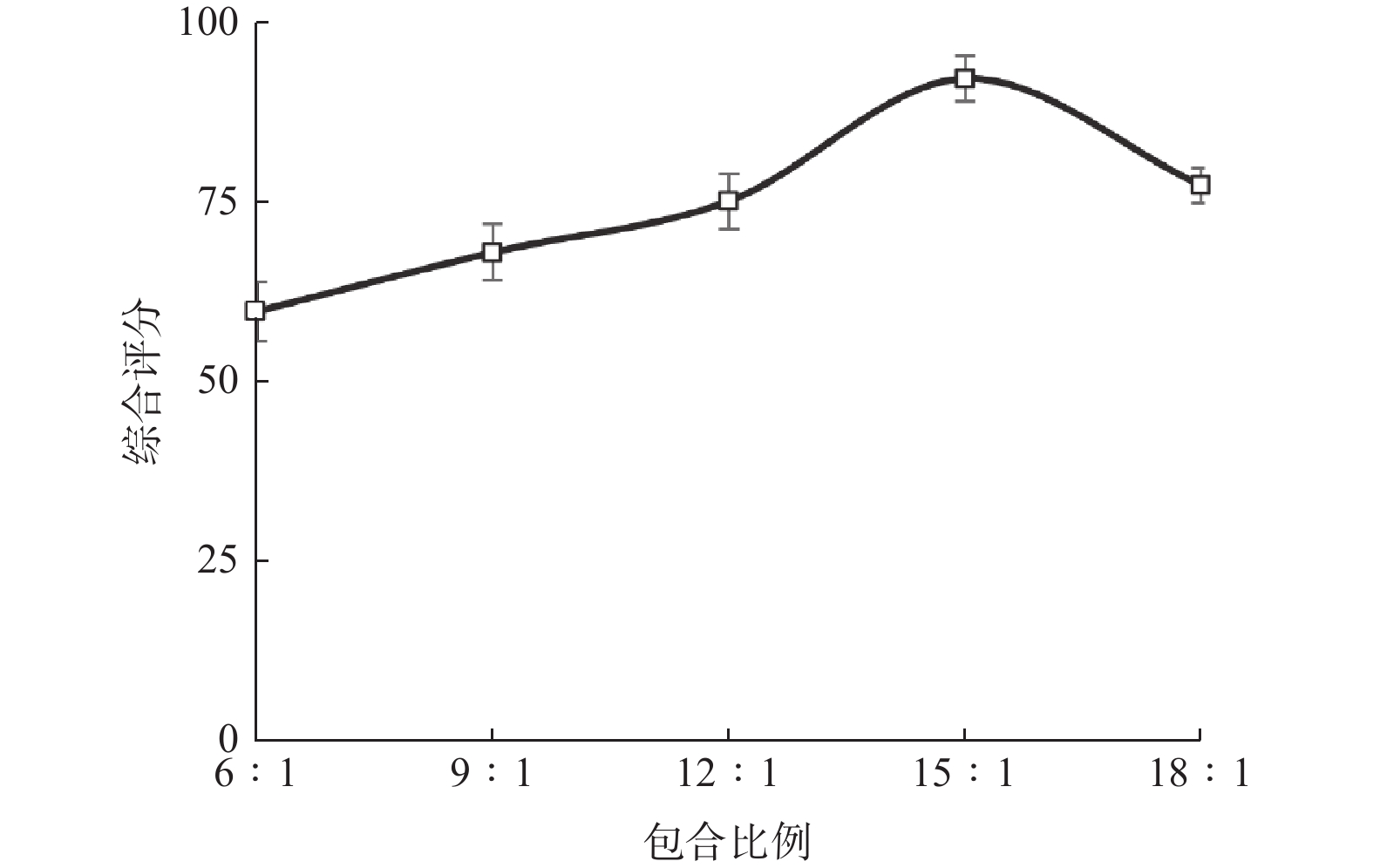
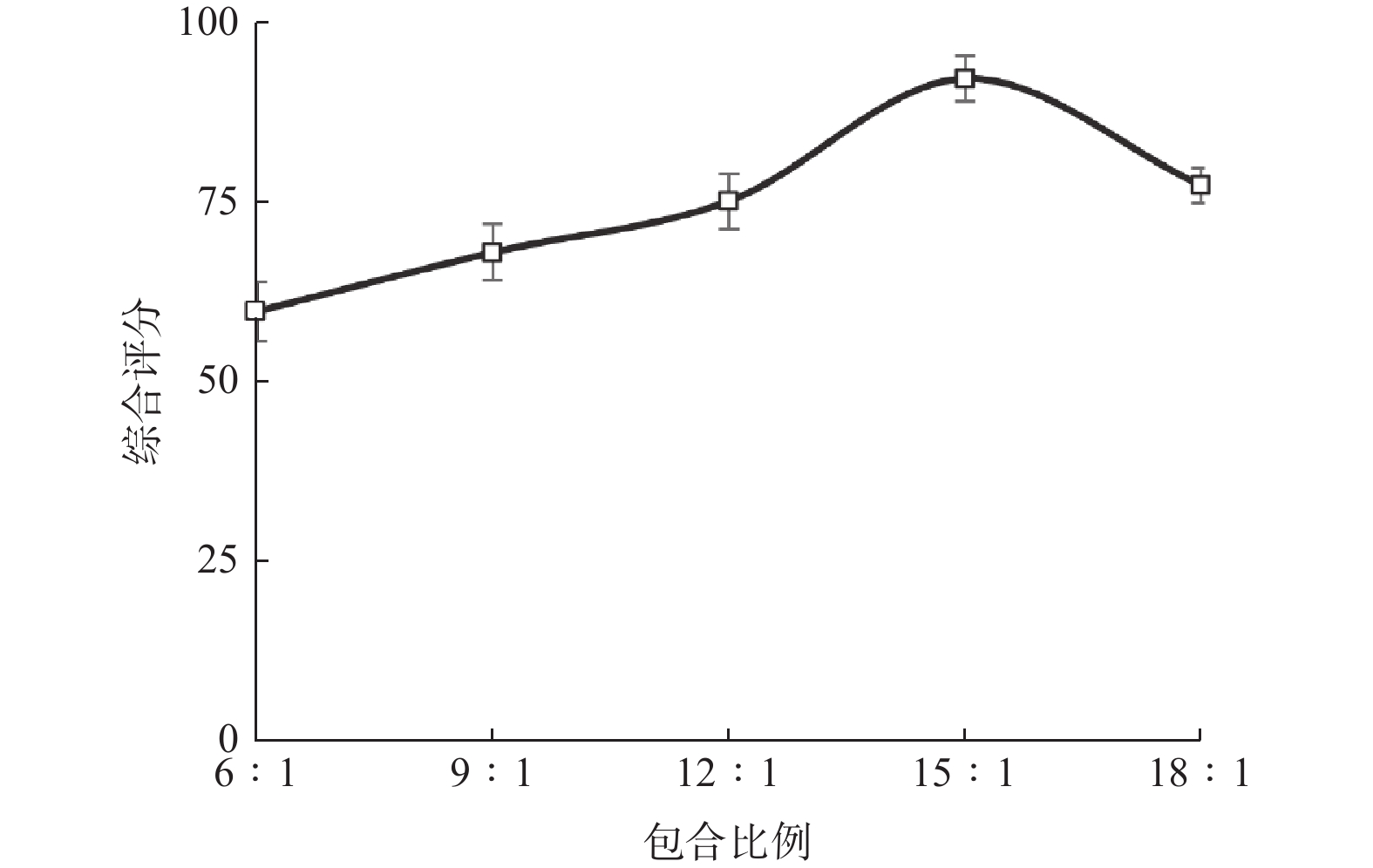
水平 包合比例 包合温度/℃ 包合时间/h −1 12∶1 50 2.0 0 15∶1 60 2.5 1 18∶1 70 3.0 表 2 响应面实验设计与结果
Table 2. Experimental design and results of response surface
实验号 A B C 含油率/% 包合率/% 收率/% 综合评分 1 −1 −1 0 27.60 17.36 69.63 79.86 2 1 −1 0 33.71 14.47 69.06 77.88 3 0 −1 1 28.59 15.97 53.05 72.84 4 −1 0 −1 39.06 19.82 69.65 94.21 5 0 0 0 29.71 14.22 61.94 72.24 6 1 0 1 21.82 13.9 68.75 67.39 7 1 0 −1 28.26 15.79 63.29 75.05 8 −1 1 0 29.64 15.28 57.50 73.33 9 0 −1 −1 33.84 16.84 71.06 83.37 10 0 1 −1 29.29 16.55 62.58 77.34 11 −1 0 1 29.31 19.16 67.00 84.50 12 0 0 0 37.31 22.21 59.40 95.36 13 0 0 0 23.31 13.40 61.52 65.37 14 1 1 0 35.92 19.31 65.76 89.55 15 0 0 0 28.3 17.91 69.19 81.52 16 0 0 0 34.42 18.71 65.05 84.84 17 0 1 1 35.4 20.70 60.46 90.79 表 3 方差分析结果
Table 3. Analysis of variance results
方差来源 平方和 自由度υ 均方 F值 P值 模型 1192.56 9 132.51 9.43 0.0037 A 36.04 1 36.04 2.57 0.1532 B 36.99 1 36.99 2.63 0.1487 C 156.78 1 156.78 11.16 0.0124 AB 26.42 1 26.42 1.88 0.2126 AC 46.52 1 46.52 3.31 0.1116 BC 2.48 1 2.48 0.18 0.6872 A2 217.31 1 217.31 15.47 0.0057 B2 129.52 1 129.52 9.22 0.0189 C2 454.78 1 454.78 32.38 0.0007 残差 98.32 7 14.05 — — 失拟项 50.19 3 16.73 1.39 0.3675 纯误差 48.13 4 12.03 — — 总计 1290.88 16 — — — -
[1] XUE Q, XIANG Z D, WANG S G, et al. Recent advances in nutritional composition, phytochemistry, bioactive, and potential applications of Syzygium aromaticum L. (Myrtaceae)[J] . Frontiers in Nutrition, 2022, 9: 1002147. doi: 10.3389/fnut.2022.1002147 [2] ZHANG P, ZHANG E L, XIAO M, et al. Enhanced chemical and biological activities of a newly biosynthesized eugenol glycoconjugate, eugenol α-D-glucopyranoside[J] . Applied Microbiology and Biotechnology, 2013, 97(3): 1043 − 1050. doi: 10.1007/s00253-012-4351-2 [3] HAN X S, PARKER T L. Anti-inflammatory activity of clove (Eugenia caryophyllata) essential oil in human dermal fibroblasts[J] . Pharmaceutical Biology, 2017, 55(1): 1619 − 1622. doi: 10.1080/13880209.2017.1314513 [4] ZHANG L F, GU B, WANG Y. Clove essential oil confers antioxidant activity and lifespan extension in C. elegans via the DAF-16/FOXO transcription factor[J] . Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 2021, 242: 108938. [5] SINGH J, BAGHOTIA A, GOEL S P. Eugenia caryophyllata thunberg (Family Myrtaceae): a review[J] . International Journal of Research in Pharmaceutical and Biomedical Sciences, 2012, 3(4): 1469 − 1475. [6] LI Z H, LI K, TENG M L, et al. Functionality-related characteristics of hydroxypropyl-β-cyclodextrin for the complexation[J] . Journal of Molecular Liquids, 2022, 365: 120105. doi: 10.1016/j.molliq.2022.120105 [7] GHARIB R, GREIGE-GERGES H, FOURMENTIN S, et al. Hydroxypropyl-β-cyclodextrin as a membrane protectant during freeze-drying of hydrogenated and non-hydrogenated liposomes and molecule-in-cyclodextrin-in- liposomes: application to trans-anethole[J] . Food Chemistry, 2018, 267: 67 − 74. doi: 10.1016/j.foodchem.2017.10.144 [8] 方浩标, 李春海, 张钟, 等. 响应面试验优化饱和水溶液法制备紫苏精油β-环糊精包合物工艺及其理化性质[J] . 绿色包装, 2019(1): 47 − 54. [9] 韩林, 张艳侠. 丁香挥发油β-环糊精包合物的制备研究[J] . 西北药学杂志, 2011, 26(6): 447 − 449. doi: 10.3969/j.issn.1004-2407.2011.06.024 [10] 李双双, 李希, 邓谦, 等. 椒香温中止痛方挥发油β-环糊精包合工艺优化及其包合物表征[J] . 中成药, 2020, 42(12): 3128 − 3134. doi: 10.3969/j.issn.1001-1528.2020.12.003 [11] 王琪, 胡佳亮, 张金良, 等. 薄荷-荆芥穗挥发油羟丙基-β-环糊精包合物的制备[J] . 中成药, 2020, 42(12): 3122 − 3128. doi: 10.3969/j.issn.1001-1528.2020.12.002 [12] 代良敏, 代良萍, 陈永钧, 等. 基于Box-Behnken实验优化地榆皂苷元固体脂质纳米粒的制备[J] . 时珍国医国药, 2023, 34(9): 2117 − 2120. doi: 10.3969/j.issn.1008-0805.2023.09.17 [13] ABD-EL-AZIZ N M, HIFNAWY M S, EL-ASHMAWY A A, et al. Application of Box-Behnken design for optimization of phenolics extraction from Leontodon hispidulus in relation to its antioxidant, anti-inflammatory and cytotoxic activities[J] . Scientific Reports, 2022, 12(1): 8829. doi: 10.1038/s41598-022-12642-2 [14] KUSHWAHA P, SHUKLA B, DWIVEDI J, et al. Validated high-performance thin-layer chromatographic analysis of curcumin in the methanolic fraction of Curcuma longa L. rhizomes[J] . Future Journal of Pharmaceutical Sciences, 2021, 7(1): 178. doi: 10.1186/s43094-021-00330-3 [15] TORIMOTO T, YAMAGUCHI N, MAEDA Y, et al. Development of plasmonic thin-layer chromatography for size-selective and optical-property-dependent separation of quantum dots[J] . NPG Asia Materials, 2022, 14(1): 64. doi: 10.1038/s41427-022-00414-3 [16] YUAN C, JIN Z Y, XU X M. Inclusion complex of astaxanthin with hydroxypropyl-β-cyclodextrin: UV, FTIR, 1H NMR and molecular modeling studies[J] . Carbohydrate Polymers, 2012, 89(2): 492 − 496. doi: 10.1016/j.carbpol.2012.03.033 [17] YUAN C, LIU B G, LIU H. Characterization of hydroxypropyl-β-cyclodextrins with different substitution patterns via FTIR, GC-MS, and TG-DTA[J] . Carbohydrate Polymers, 2015, 118: 36 − 40. doi: 10.1016/j.carbpol.2014.10.070 [18] HAO X C, SUN X D, ZHU H Z, et al. Hydroxypropyl-β-cyclodextrin-complexed resveratrol enhanced antitumor activity in a cervical cancer model: in vivo analysis[J] . Frontiers in Pharmacology, 2021, 12: 573909. doi: 10.3389/fphar.2021.573909 -





 下载:
下载: