Thermodynamic analysis of product distribution and conversion rate of CH4/CO2 reforming products
-
摘要: 基于吉布斯自由能最小化原理,采用HSC Chemistry软件对CH4/CO2重整反应过程进行热力学分析,系统探讨反应温度、压力、进料比(n(CH4)/n(CO2)物质的量比)、O2进量和H2O进量等关键参数对系统平衡时各产物分布及反应物转化率的影响规律。研究表明,当温度为
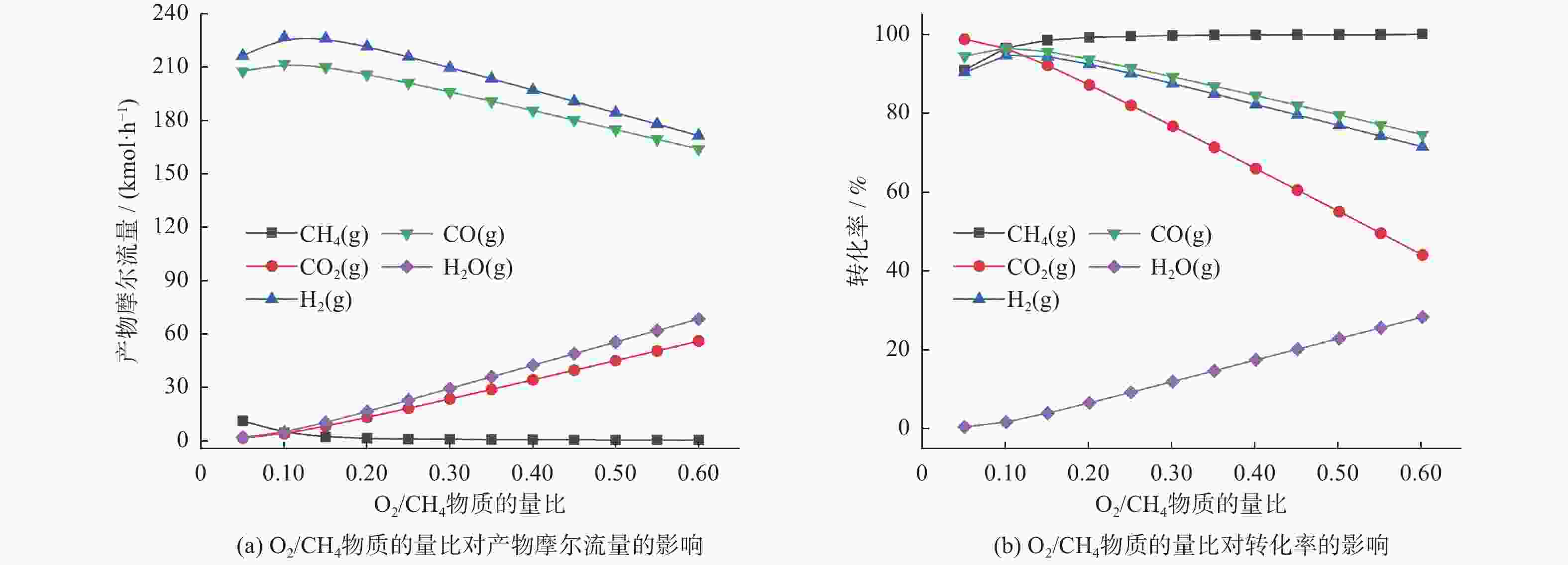
1137 K,压力为101.325 kPa,n(CH4)/n(CO2)为1.20,n(O2)/n(CH4)为0.10和n(H2O)/n(CH4)为0.07时,最有利于CH4/CO2的转化和H2/CO的生成。CH4/CO2重整作为许多化学合成链中的上游反应,能有效利用CH4和CO2两大温室气体,生产了一种重要的化学原料——合成气(H2/CO)。研究结果不仅有利于相关工业的发展,而且对全球气候的可持续发展提供有益参考。Abstract: Based on the principle of Gibbs free energy minimization using HSC chemistry software, thermodynamic analysis of the CH4/CO2 reforming reaction process was performed. The effects of key parameters such as reaction temperature, pressure, feed ratio (n(CH4)/n(CO2) molar ratio), the inlet amounts of O2 and H2O, on the the equilibrium product distribution and reactant conversion were systematically explored. The results indicate that the conditions of1137 K, 101.325 kPa, n(CH4)/n(CO2) = 1.20, n(O2)/n(CH4) = 0.10, and n(H2O)/n(CH4) = 0.07 are most favorable for CH4/CO2 conversion and H2/CO generation. As an upstream reaction in numerous chemical synthesis chains, CH4/CO2 reforming effectively utilizes the two major greenhouse gases CH4 and CO2 to produce syngas (H2/CO), an important chemical raw materia. These findings benefits the development of related industries and provide a useful reference for global climate sustainability.-
Key words:
- CH4/CO2 /
- reforming /
- thermodynamics /
- conversion rate /
- equilibrium
-
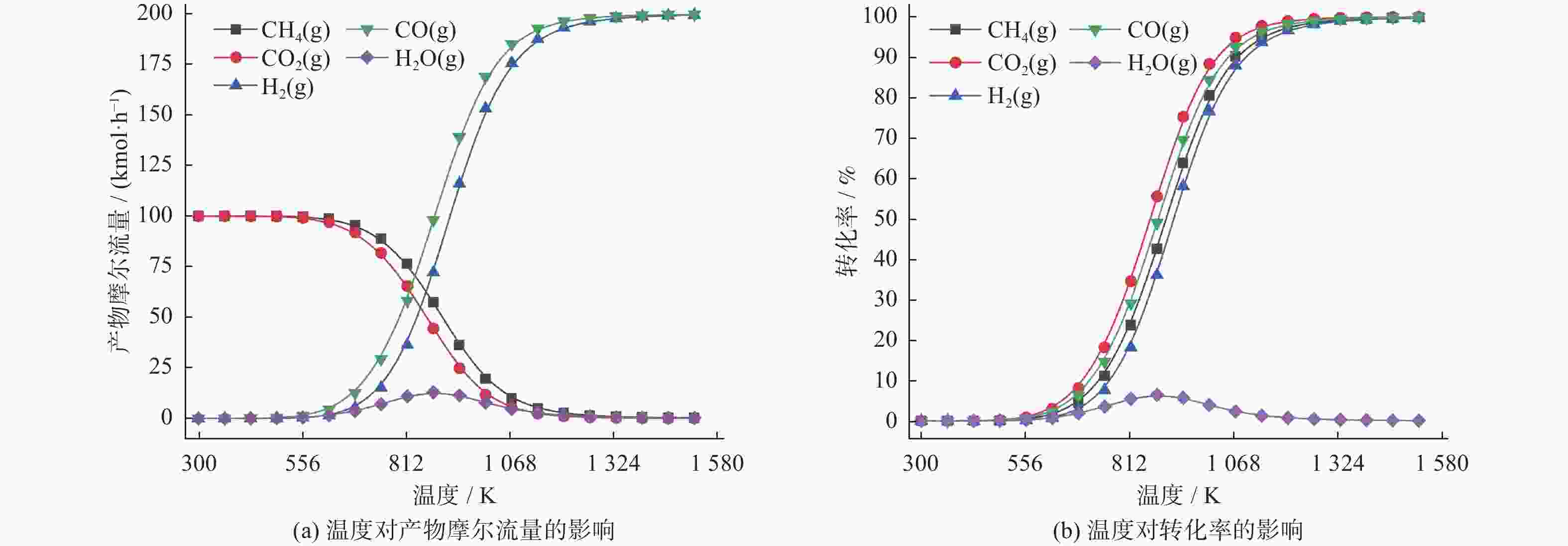
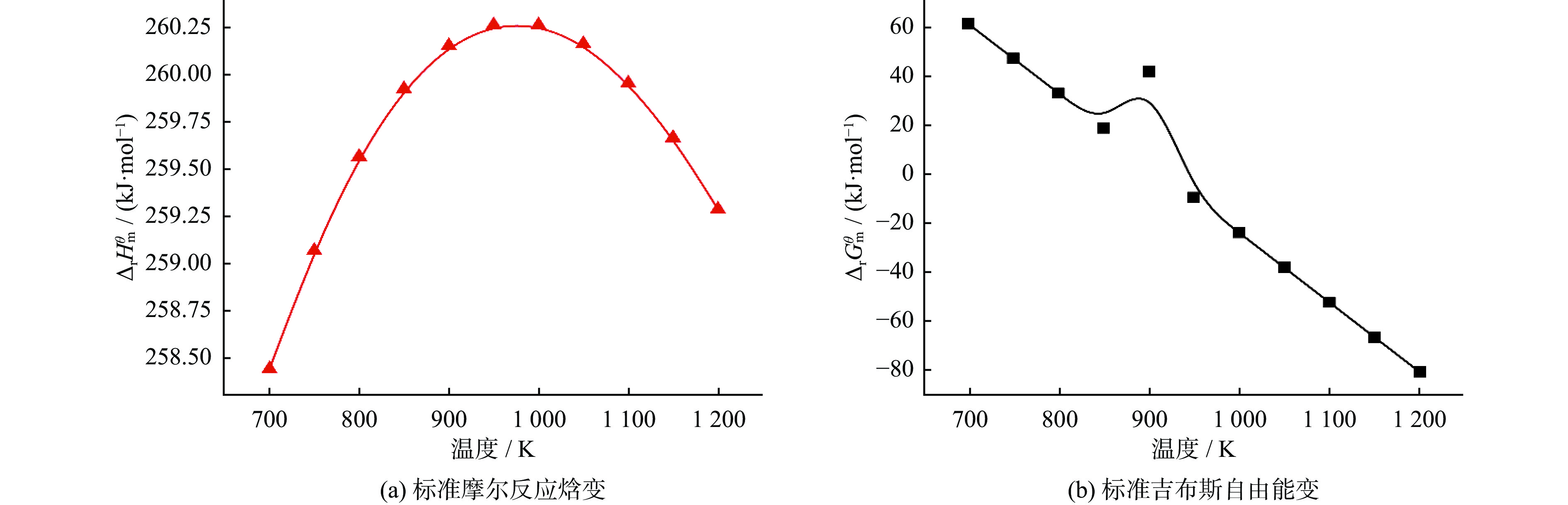
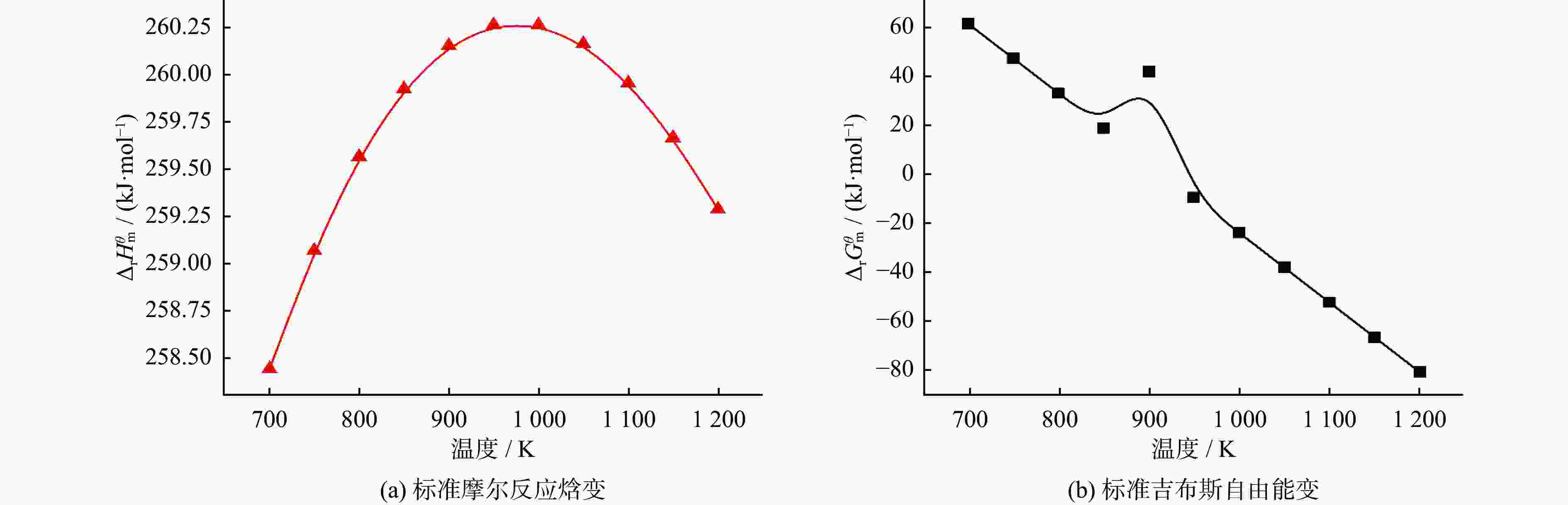
表 1 温度对物质摩尔流量及转化率的影响
Table 1. Effect of temperature on molar flow rate and conversion rate of substances
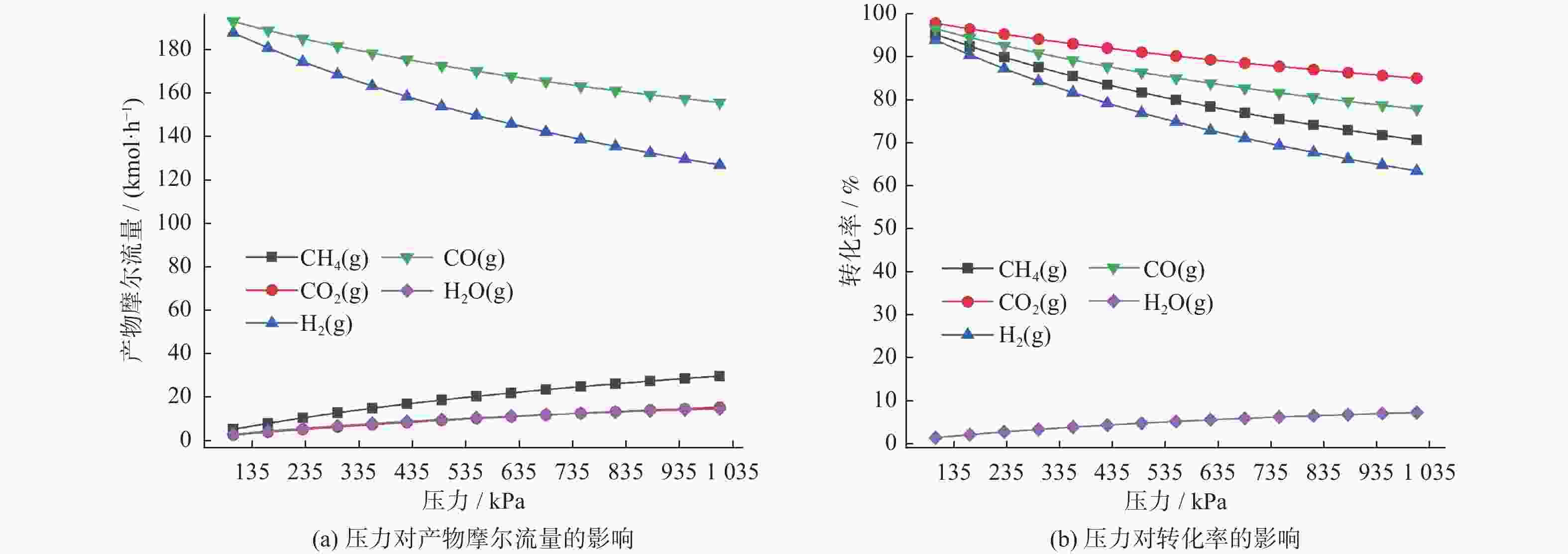
物质 摩尔流量变化 转化率变化 T<622 K 622 K≤T< 1137 KT= 1137 KT<622 K 622 K≤T< 1137 KT= 1137 KCH4 不变 下降 基本稳定 缓慢增加 急剧增长 基本稳定 CO2 不变 下降 基本稳定 缓慢增加 急剧增长 基本稳定 CO 不变 上升 基本稳定 缓慢增加 急剧增长 基本稳定 H2 不变 上升 基本稳定 缓慢增加 急剧增长 基本稳定 H2O 不变 上升 基本稳定 缓慢增加 缓慢增长,879 K后开始下降 基本稳定 表 2 压力对物质摩尔流量及转化率的影响
Table 2. Effect of pressure on molar flow rate and conversion rate of substances
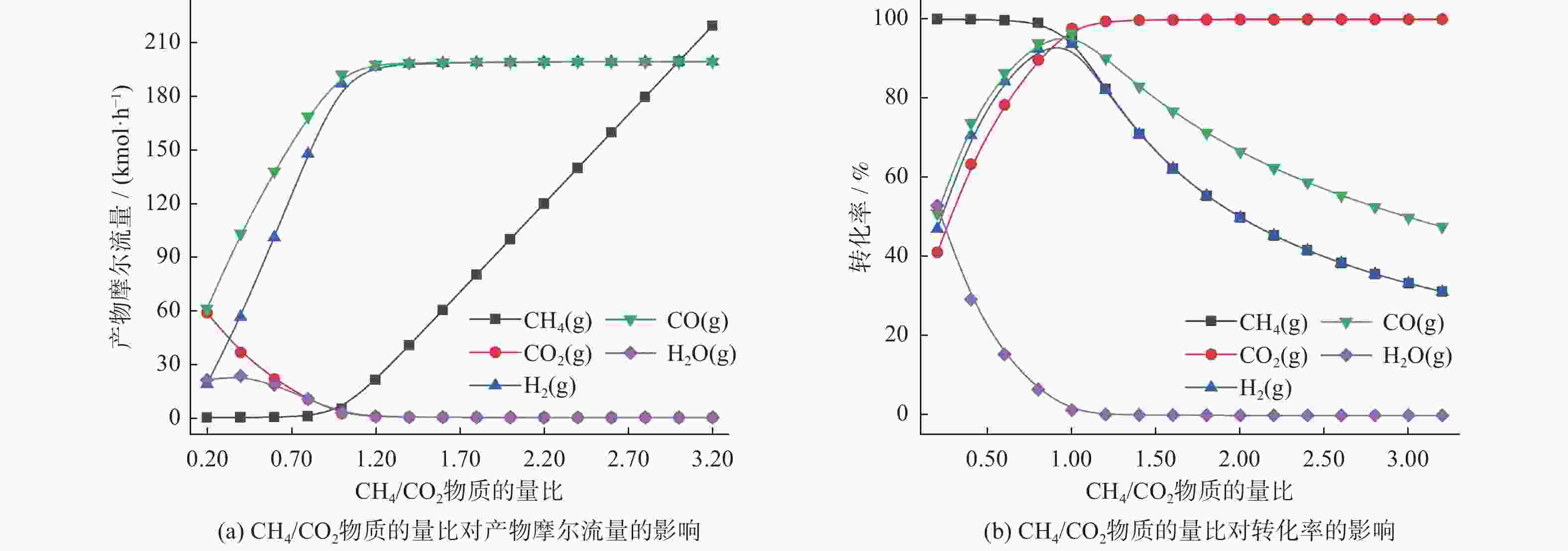
物质 p=100 kPa p=1 010 kPa 摩尔流量/(kmol·h−1) 转化率/% 摩尔流量/(kmol·h−1) 转化率/% CH4 4.89 95.10 29.45 70.55 CO2 2.29 97.71 15.09 84.91 CO 192.81 96.41 155.46 77.73 H2 187.60 93.20 126.72 63.36 H2O 2.61 1.30 14.37 7.18 表 3 CH4/CO2物质的量比对物质摩尔流量及转化率的影响
Table 3. Effect of CH4/CO2 molar ratio on molar flow rate and conversion rate
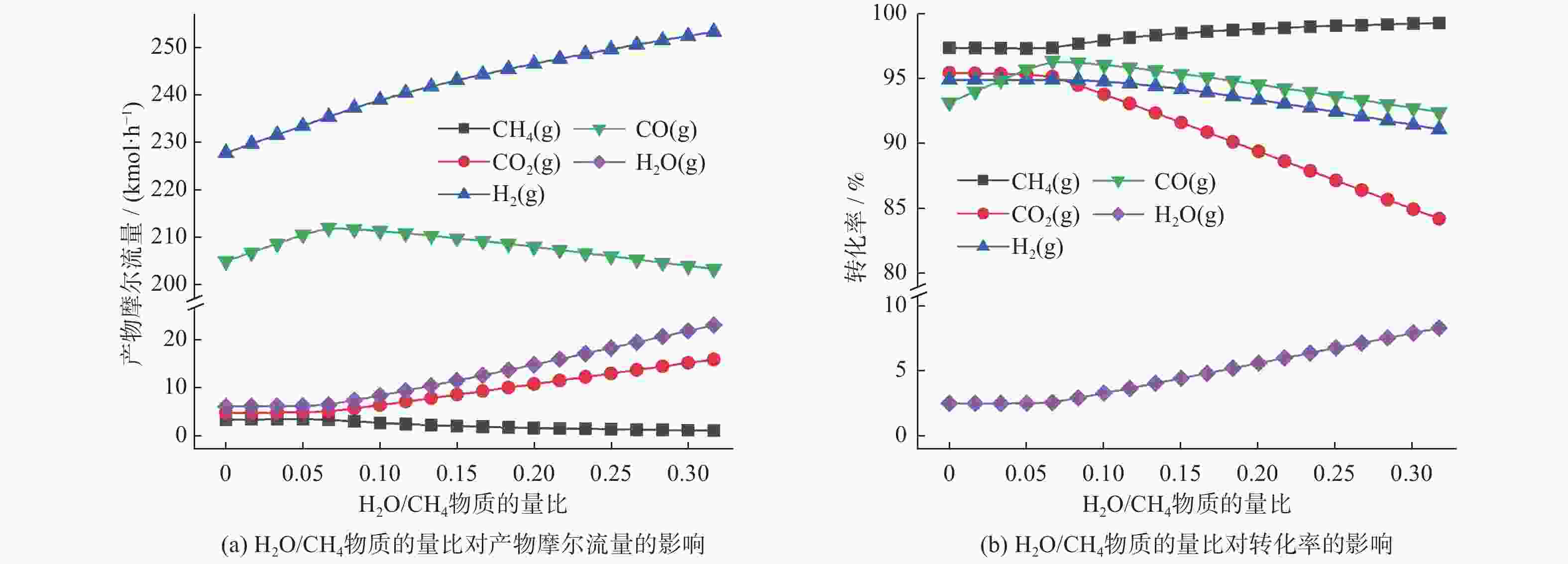
物质 摩尔流量变化情况 转化率变化情况 CH4 n(CH4)/n(CO2)为0.20 ~ 1.00时基本不变;之后急剧上升 n(CH4)/n(CO2)为0.20 ~ 0.80内基本不变;之后开始下降 CO2 n(CH4)/n(CO2)为0.20 ~ 1.00时急剧下降;比值为1.20后达到稳定 n(CH4)/n(CO2)为0.20 ~ 0.80时急剧上升;比值为1.20后趋于稳定 CO n(CH4)/n(CO2)为0.20 ~ 1.00时急剧上升;比值为1.20后达到稳定 先增后减,在n(CH4)/n(CO2)为1.00时达到最大值 H2 n(CH4)/n(CO2)为0.20 ~ 1.00时急剧上升;比值为1.20后达到稳定 先增后减,在n(CH4)/n(CO2)为1.00时达到最大值 H2O n(CH4)/n(CO2)为0.20 ~ 0.60时先增后减;比值为0.40时达到最大;比值为1.20后达到稳定 n(CH4)/n(CO2)为0.20 ~ 1.00时急剧减少;比值为1.20后趋于稳定 -
[1] LE PHUONG D H, ALSAIARI M, PHAM C Q, et al. Carbon dioxide reforming of methane over modified iron-cobalt alumina catalyst: role of promoter[J] . Journal of the Taiwan Institute of Chemical Engineers, 2024, 155: 105253. doi: 10.1016/j.jtice.2023.105253 [2] ZHANG M, ZHANG J F, ZHOU Z L, et al. Effects of the surface adsorbed oxygen species tuned by rare-earth metal doping on dry reforming of methane over Ni/ZrO2 catalyst[J] . Applied Catalysis B: Environmental, 2020, 264: 118522. [3] JOO S, KIM K, KWON O, et al. Enhancing thermocatalytic activities by upshifting the d-band center of exsolved Co-Ni-Fe ternary alloy nanoparticles for the dry reforming of methane[J] . Angewandte Chemie International Edition, 2021, 60(29): 15912 − 15919. [4] CHAWL S K, GEORGE M, PATEL F, et al. Production of synthesis gas by carbon dioxide reforming of methane over nickel based and perovskite catalysts[J] . Procedia Engineering, 2013, 51: 461 − 466. doi: 10.1016/j.proeng.2013.01.065 [5] AZIZ M A A, SETIABUDI H D, TEH L P, et al. A review of heterogeneous catalysts for syngas production via dry reforming[J] . Journal of the Taiwan Institute of Chemical Engineers, 2019, 101: 139 − 158. [6] 张哲. 我国可再生能源法律完善研究[D] . 杨凌: 西北农林科技大学, 2009. [7] 钱慧琳, 冉金玲, 何安帮, 等. 二氧化碳-甲烷干气重整反应及其积炭控制的热力学分析[J] . 低碳化学与化工, 2023, 48(5): 55 − 61. [8] 李建伟, 陈冲, 王丹, 等. 甲烷二氧化碳重整热力学分析[J] . 石油与天然气化工, 2015, 44(3): 60 − 64. [9] CHEIN R Y, CHEN Y C, YU C T, et al. Thermodynamic analysis of dry reforming of CH4 with CO2 at high pressures[J] . Journal of Natural Gas Science and Engineering, 2015, 26: 617 − 629. [10] CHEN Y, ZHANG Y M, FAN G Z, et al. Cooperative catalysis coupling photo-/photothermal effect to drive Sabatier reaction with unprecedented conversion and selectivity[J] . Joule, 2021, 5(12): 3235 − 3251. doi: 10.1016/j.joule.2021.11.009 [11] FAN L, ZHAO Y L, CHEN L, et al. Selective production of ethylene glycol at high rate via cascade catalysis[J] . Nature Catalysis, 2023, 6(7): 585 − 595. doi: 10.1038/s41929-023-00977-6 [12] TANG X Y, YANG W W, MA X, et al. Synergistic enhancement of reaction and separation for a solar membrane reactor by topology optimization of catalyst bed[J] . Chemical Engineering Journal, 2023, 472: 145123. doi: 10.1016/j.cej.2023.145123 [13] TANG X Y, YANG W W, MA X, et al. An integrated modeling method for membrane reactors and optimization study of operating conditions[J] . Energy, 2023, 268: 126730. doi: 10.1016/j.energy.2023.126730 [14] 石天宝, 张秋菊, 纪容昕, 等. CH4和CO2转化催化剂的研究[J] . 化工生产与技术, 2000, 7(2): 12 − 15. [15] 傅献彩, 沈文霞, 姚天扬, 等. 物理化学[M] . 5版. 北京: 高等教育出版社, 2005. [16] 张迪茜. 生物质能源研究进展及应用前景[D] . 北京: 北京理工大学, 2015. -





 下载:
下载: