Research on Enhanced catalytic soot combustion performance over NiO/Co3O4 within mesoporous nanosheets
-
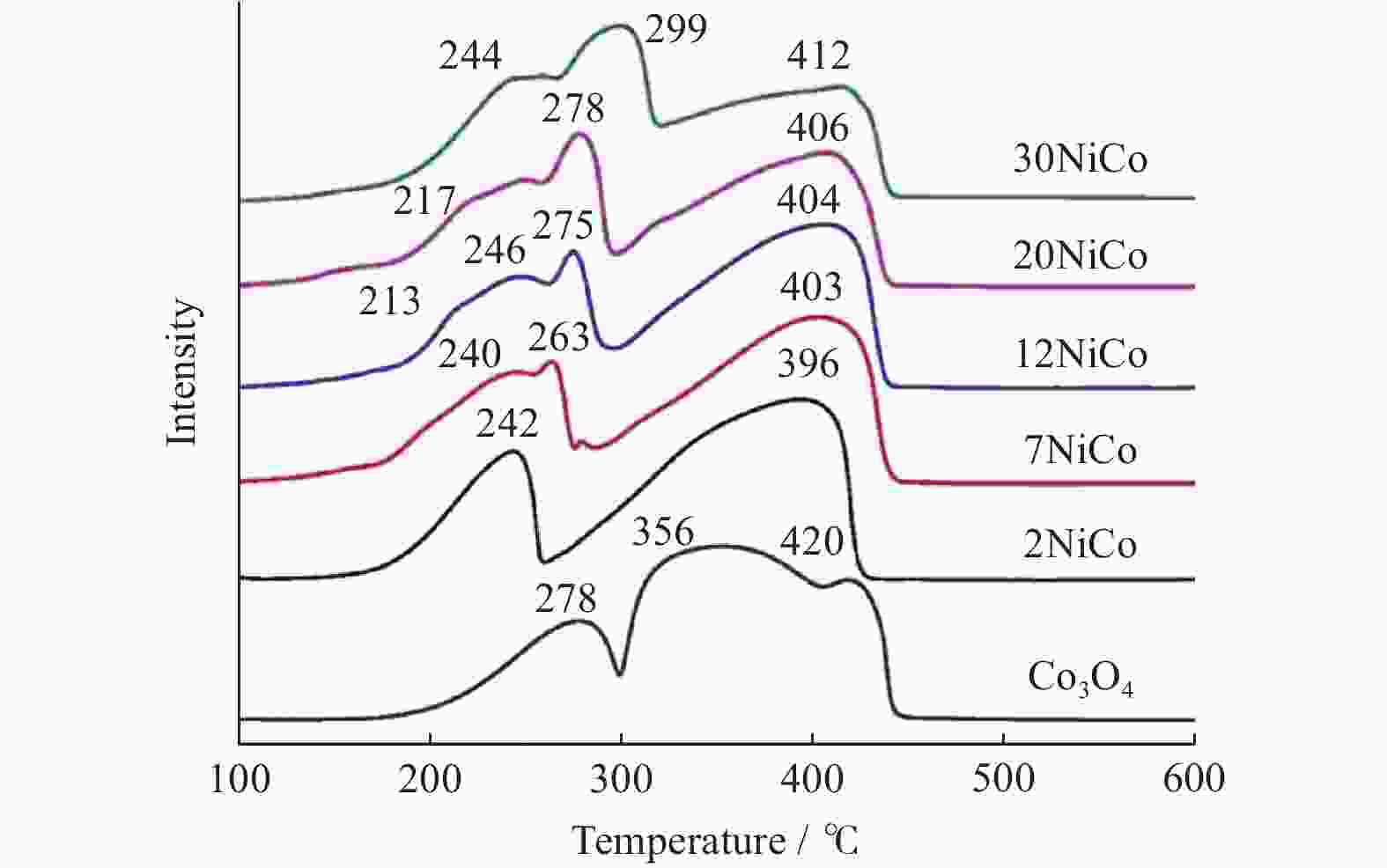
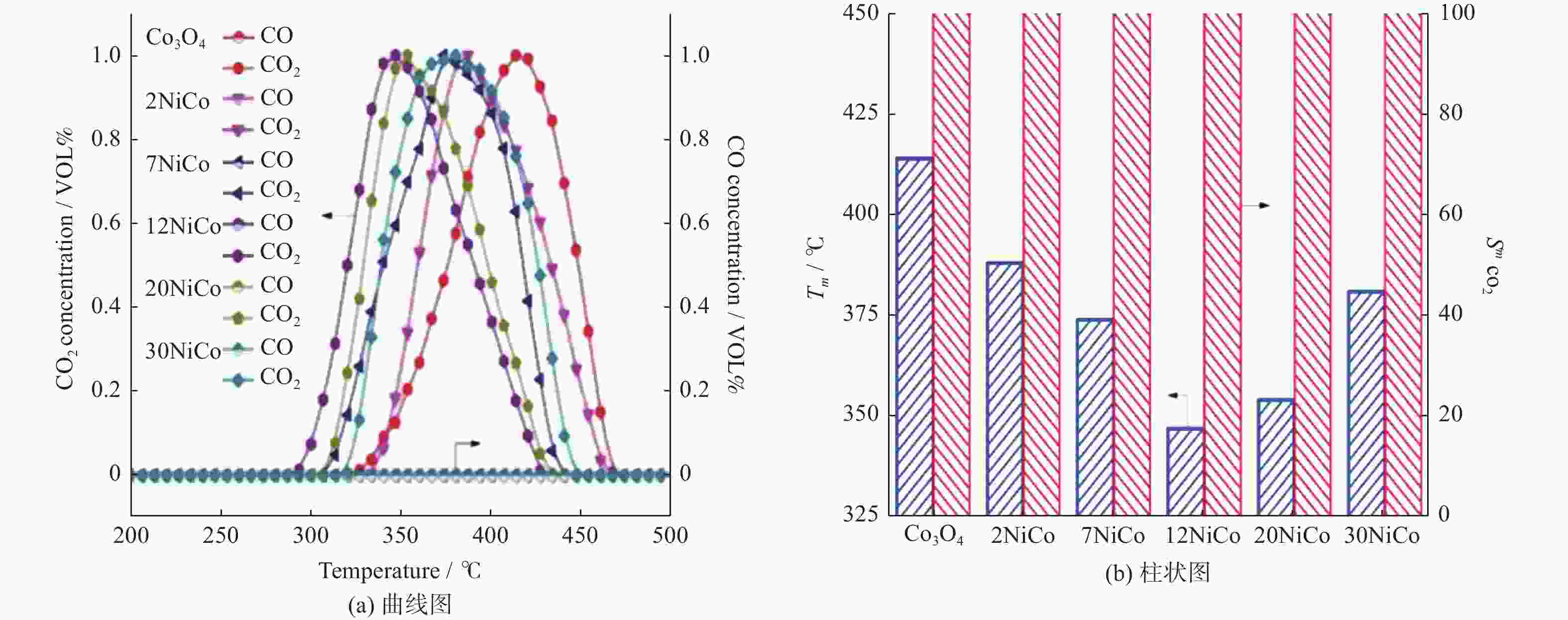
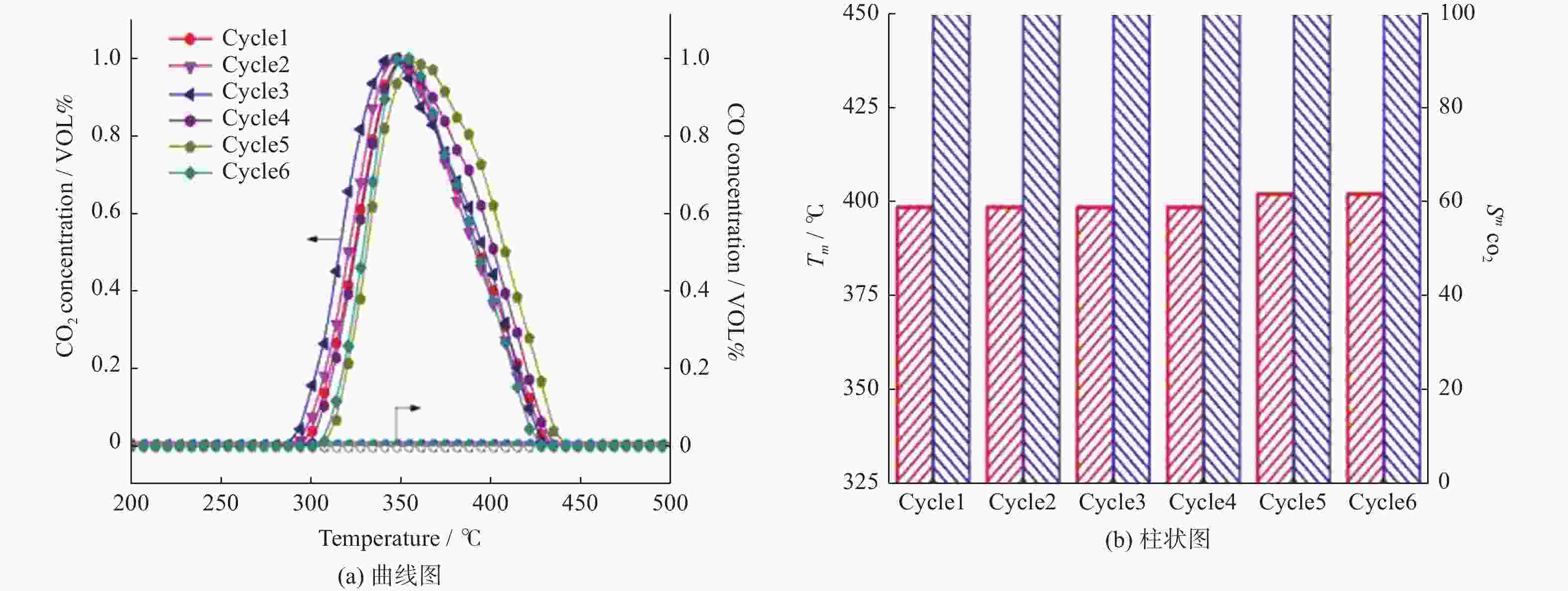
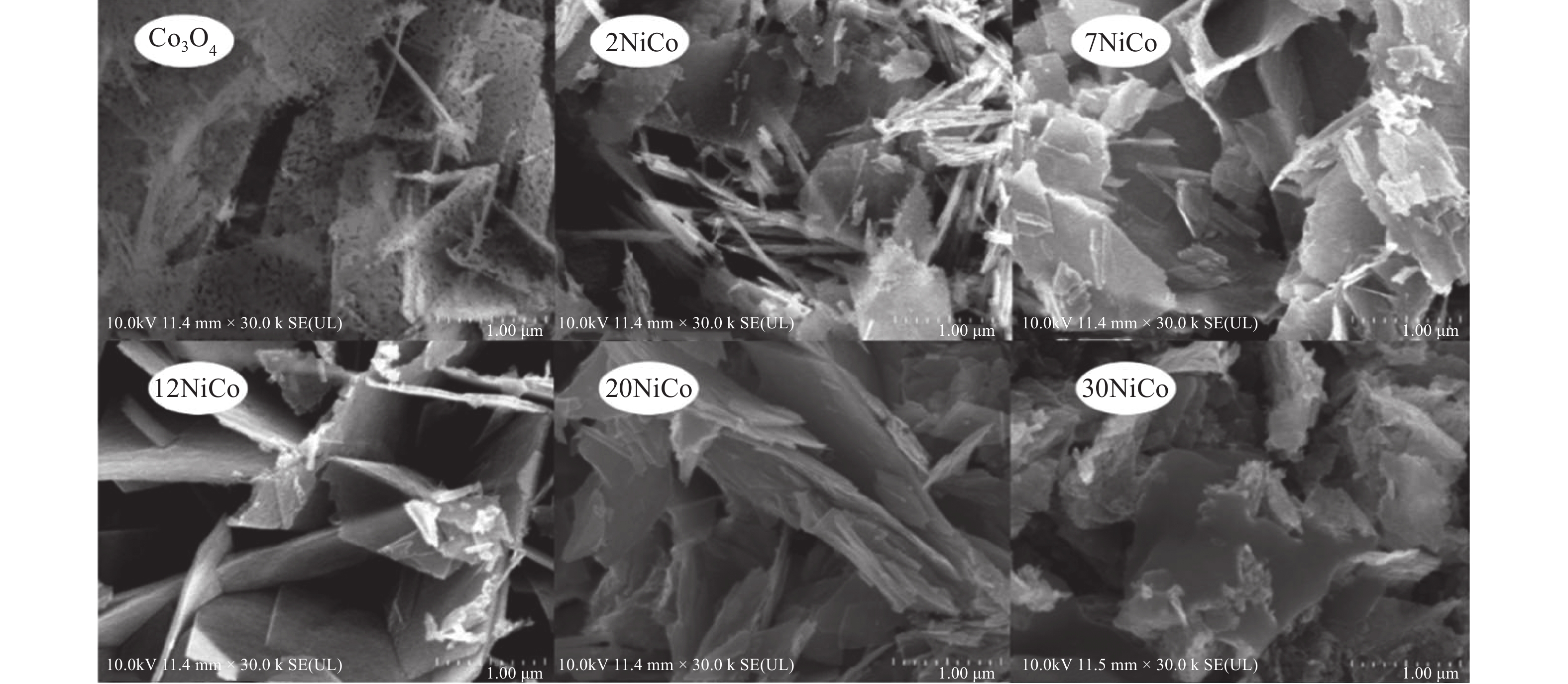
摘要: 采用水热结合等体积浸渍法制备一系列NiO/Co3O4介孔纳米片催化剂,并以柴油机碳烟催化燃烧为模型反应评价其催化性能. 研究表明,当Ni/Co物质的量比为12%时,所制备的催化剂12NiCo具有最佳碳烟颗粒催化燃烧活性,其Tm为347 ℃,CO2选择性为100%,主要归因于以下原因:1)二维片状结构及其较高的比表面积有效增大了催化剂与碳烟颗粒的接触界面;2)纳米片具有丰富的介孔孔道,有利于降低传质阻力,进而促进气体反应物的吸附与扩散;3)NiO的引入增强了催化剂的氧化还原能力,促进了氧物种的吸附与活化生成活性氧物种,同时也促进了NO氧化形成氧化能力更强的NO2参与反应,进一步提升催化活性. 此外,该催化剂12NiCo具有良好的循环使用性能,显示了一定的潜在实用价值.Abstract: A series of NiO/Co3O4 catalysts within mesoporous nanosheets, synthesized by a facile hydrothermal combining wet-impregnation route, have been developed to catalyze diesel soot combustion. Results attest that, when the molar ratio of Ni/Co reached 12%, the catalyst of 12NiCo exhibited the optimal catalytic soot combustion performance, giving Tm of 347 ℃ and 100% CO2 selectivity, which can be chiefly credited to the synergetic effect of the following factors. Firstly, the unique 2D nanosheets together with high surface area enlarged the contact interface of catalyst-soot particles. Secondly, abundant mesopores within nanosheets favored the significant decrease in mass transfer resistance and then in turn promoted the adsorption and diffusion of gas reactants. Thirdly, the enhanced reducibility by introducing NiO not merely facilitated the adsorption and activation of oxygen species to form active oxygen species, but also benefited NO oxidation to produce NO2 with higher oxidative capability, thereby improving catalytic soot combustion performance remarkably. In addition, 12NiCo also presented the excellent reusability, demonstrating good potential in future practical applications.
-
Key words:
- diesel exhaust /
- soot particles /
- NiO/Co3O4 /
- mesoporous nanosheets /
- catalytic combustion
-
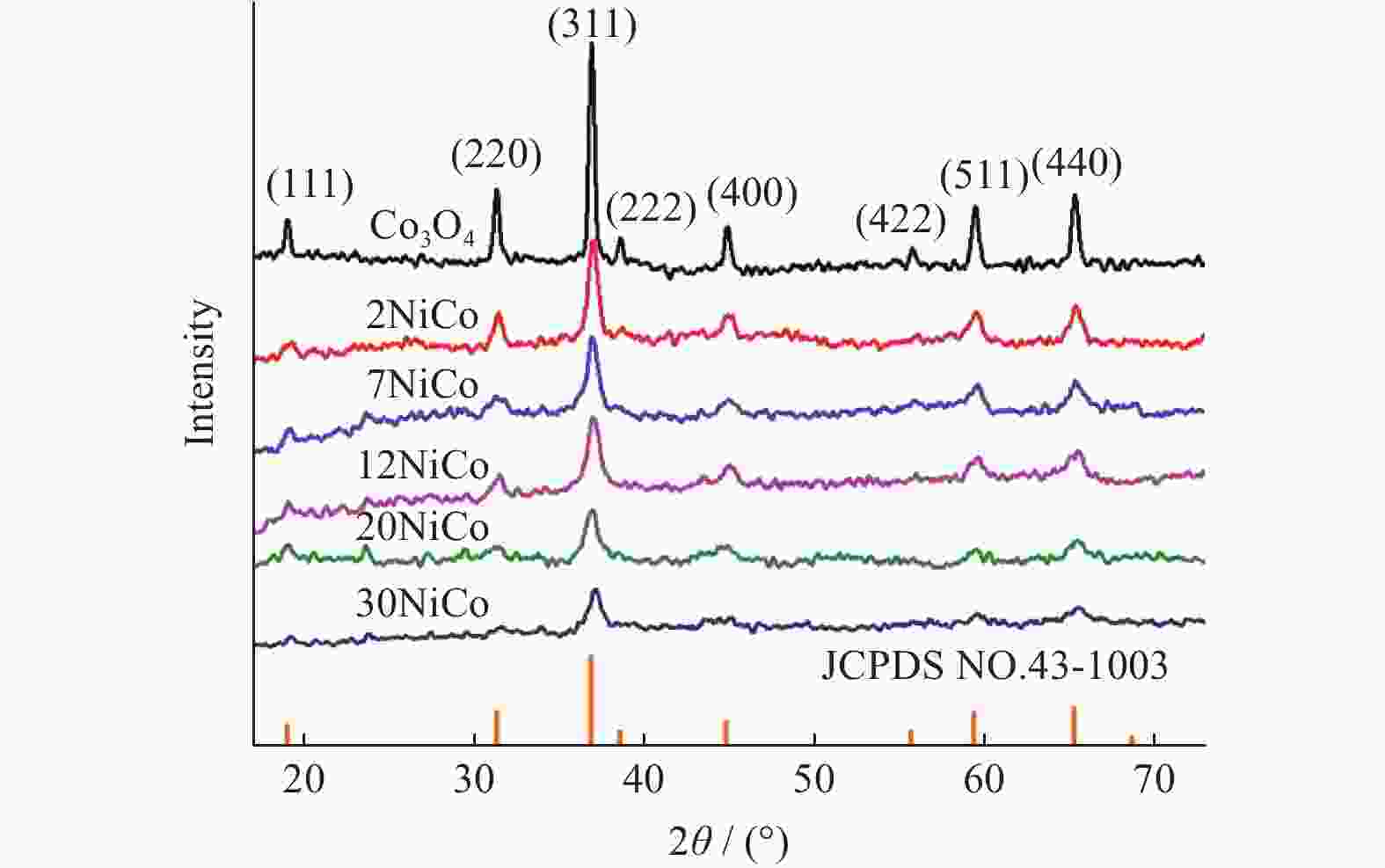
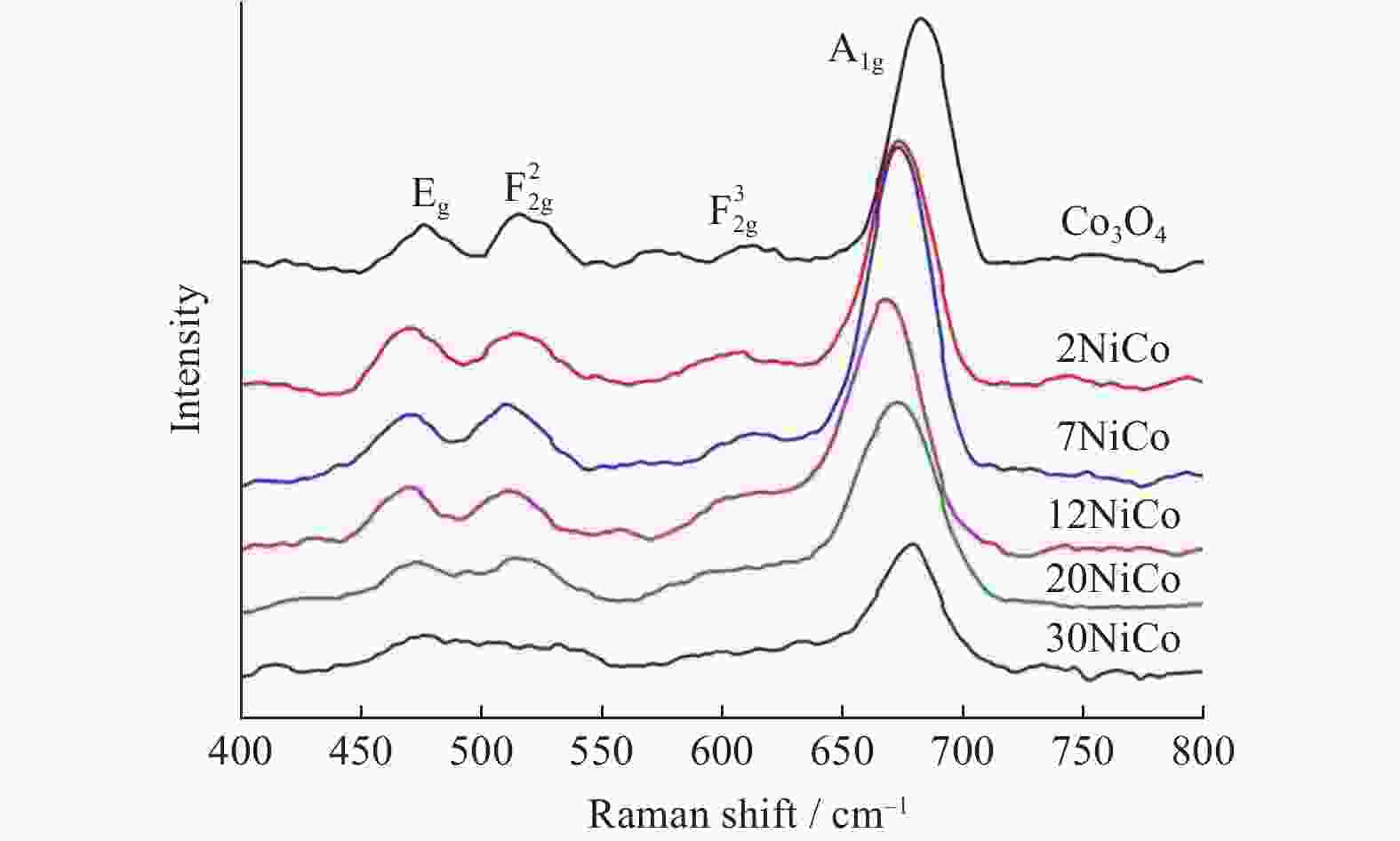
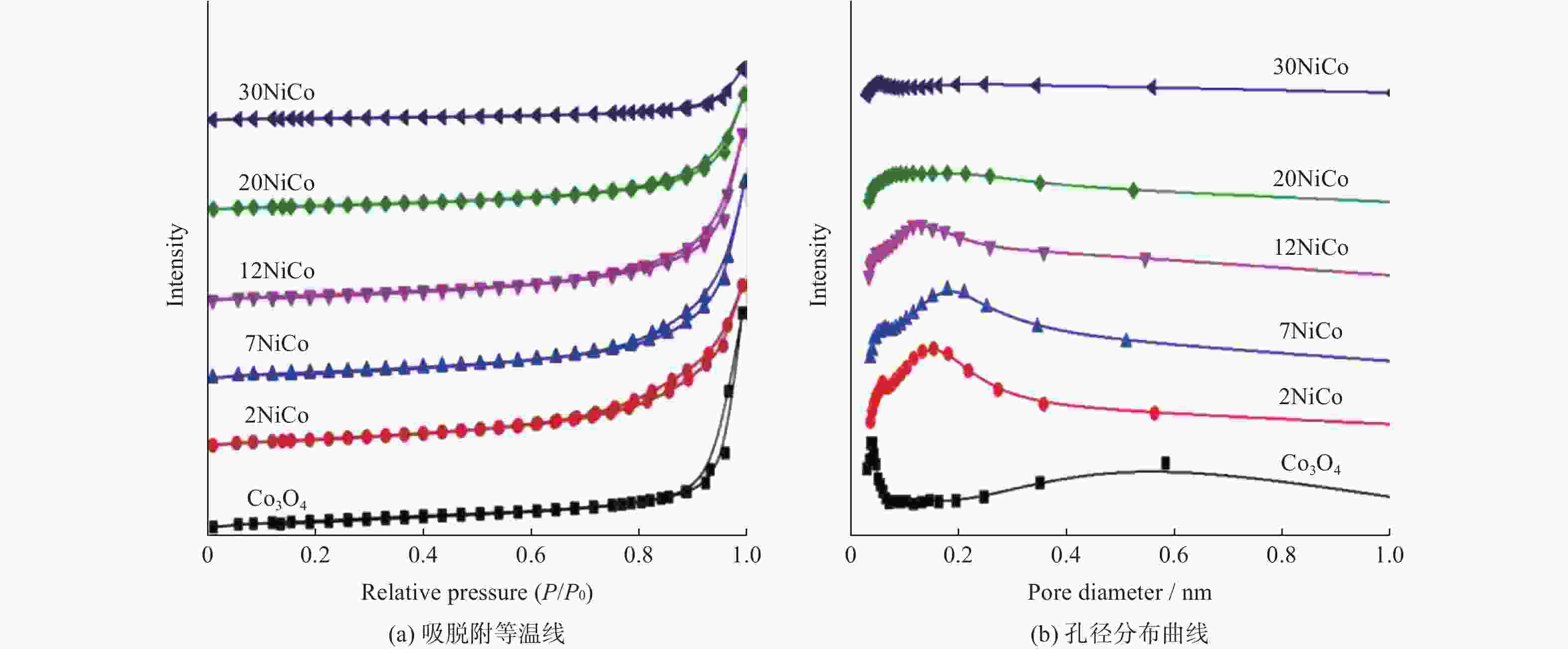
表 1 不同催化剂的物理结构参数及H2消耗量
Table 1. Physicochemical parameters and H2 consumption of different catalysts
Catalyst SBET/
(m2•g−1)VP/
(cm3•g−1)DP/
nmCrystallize size/
nmH2 consumption/
(mmol•g−1)Co3O4 20 0.094 24 29.0 12.1 2NiCo 19 0.091 22 15.4 12.4 7NiCo 17 0.087 20 13.0 12.5 12NiCo 15 0.074 19 12.1 12.8 20NiCo 11 0.051 18 10.9 12.6 30NiCo 9 0.022 15 10.3 12.5 表 2 不同催化剂的元素组成及价态参数
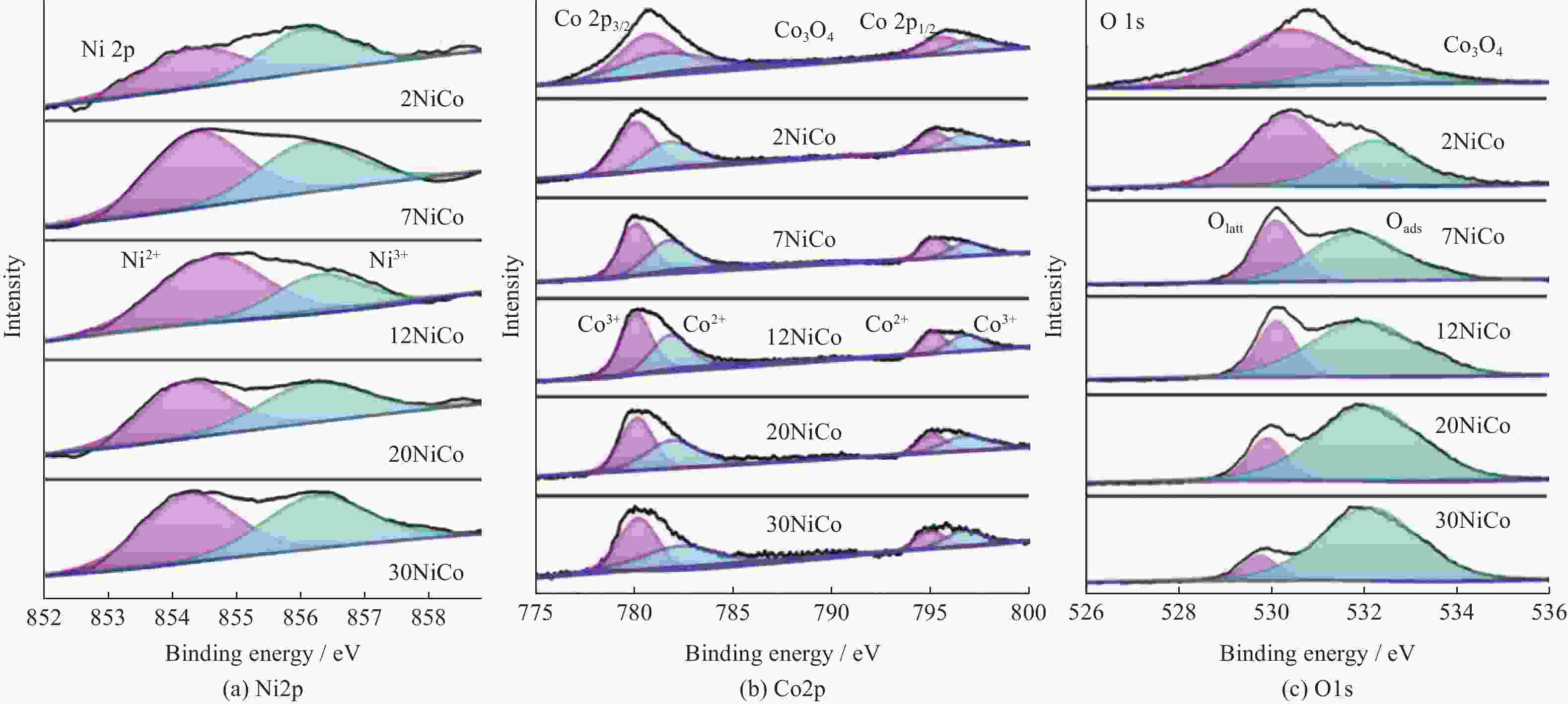
Table 2. Elemental composition and chemical valence states of different catalysts
Catalyst Ni•2p Co•2p O•1s Ni2+ /% Ni3+ /% Ni3+ /Ni2+ Co2+ /% Co3+ /% Co3+ /Co2+ Oads /% Olatt /% Oads /Olatt Co3O4 — — — 48 52 1.08 25 75 0.33 2NiCo 51 49 0.96 47 53 1.13 40 60 0.67 7NiCo 60 40 0.67 45 55 1.22 49 51 0.96 12NiCo 67 33 0.49 42 58 1.38 63 37 1.70 20NiCo 55 45 0.81 46 54 1.17 84 16 5.25 30NiCo 52 48 0.92 50 50 1.00 90 10 9.0 -
[1] 贺泓, 翁端, 资新运. 柴油车尾气排放污染控制技术综述[J] . 环境科学,2007,28(6):1169 − 1177. doi: 10.3321/j.issn:0250-3301.2007.06.001 [2] 罗明. 柴油机尾气排放控制技术分析[J] . 中国新技术新产品,2014(14):34. [3] PIUMETTI M, BENSAID S, RUSSO N, et al. Nanostructured ceria-based catalysts for soot combustion: investigations on the surface sensitivity[J] . Applied Catalysis B:Environmental,2015,165:742 − 751. doi: 10.1016/j.apcatb.2014.10.062 [4] 李炳章, 张文军, 张园园, 等. 柴油车尾气净化技术研究进展[J] . 山东化工,2019,48(9):105 − 106. doi: 10.3969/j.issn.1008-021X.2019.15.042 [5] LI P Y, FENG L, YUAN F L, et al. Effect of surface copper species on NO+CO reaction over xCuO-Ce0.9Zr0.1O2 catalysts: in situ DRIFTS studies[J] . Catalysts,2016,6:124. doi: 10.3390/catal6080124 [6] CUI B, ZHOU L J, LI K, et al. Holey Co-Ce oxide nanosheets as a highly efficient catalyst for diesel soot combustion[J] . Applied Catalysis B:Environmental,2020,267:118670. doi: 10.1016/j.apcatb.2020.118670 [7] CHEN Y, FAN Z X, ZHANG Z C, et al. Two-dimensional metal nanomaterials: Synthesis, properties, and applications[J] . Chemical Reviews,2018,118:6409 − 6455. doi: 10.1021/acs.chemrev.7b00727 [8] DONG R H, ZHANG T, FENG X L. Interface-assisted synthesis of 2D materials: trend and challenges[J] . Chemical Reviews,2018,118:6189 − 6235. doi: 10.1021/acs.chemrev.8b00056 [9] JIAN S Q, YANG Y X, REN W, et al. Kinetic analysis of morphologies and crystal planes of nanostructured CeO2 catalysts on soot oxidation[J] . Chemical Engineering Science,2020,226:115891. doi: 10.1016/j.ces.2020.115891 [10] ANEGGI E, WIATER D, CARLA D L, et al. Shape-dependent activity of ceria in soot combustion[J] . ACS Catalysis,2013,4:172 − 181. [11] WANG M, ZHANG Y, YU Y B, et al. Synergistic effects of multicomponents produce outstanding soot oxidation activity in a Cs/Co/MnOx catalyst[J] . Environmental Science Technology,2021,55:240 − 248. doi: 10.1021/acs.est.0c06082 [12] LABCHIR N, HANNOUR A, ABDERRAHIM A, et al. Enhanced magnetic properties of magneto-electrodeposited Co and Ni nanowires[J] . Current Applied Physics,2021,25:33 − 40. doi: 10.1016/j.cap.2021.02.010 [13] MOHAMED I A. Assessment of using carbon soot as economic adsorbing material for the removal of cobalt (II) from aqueous solution[J] . Main Group Chemistry,2014,13:353 − 362. doi: 10.3233/MGC-140147 [14] LIU J, ZHAO Z, WANG J Q, et al. The highly active catalysts of nanometric CeO2-supported cobalt oxides for soot combustion[J] . Applied Catalysis B:Environmental,2008,84:185 − 195. doi: 10.1016/j.apcatb.2008.03.017 [15] ARANTXA D Q, MIRIAM N G, DOLORES L C, et al. Role of hydroxyl groups in the preferential oxidation of CO over copper oxide-cerium oxide catalysts[J] . ACS Catalysis,2016,6:1723 − 1731. doi: 10.1021/acscatal.5b02741 [16] LAI H W, WU Q, ZHAO J, et al. Mesostructured NiO/Ni composites for high-performance electrochemical energy storage[J] . Energy & Environmental Science,2016,9:2053 − 2060. [17] LE T A, KIM M S, LEE S H, et al. CO and CO2 methanation over supported Ni catalysts[J] . Catalysis Today,2017,293-294:89 − 96. doi: 10.1016/j.cattod.2016.12.036 [18] ZHAI G J, WANG J G, CHEN Z M, et al. Boosting soot combustion efficiency of Co3O4 nanocrystals via tailoring crystal facets[J] . Chemical Engineering Journal,2018,337:488 − 498. doi: 10.1016/j.cej.2017.12.141 [19] ZHAI G J, WANG J G, CHEN Z M, et al. Highly enhanced soot oxidation activity over 3DOM Co3O4-CeO2 catalysts by synergistic promoting effect[J] . Journal of Hazardous Materials,2019,363:214 − 226. doi: 10.1016/j.jhazmat.2018.08.065 [20] MARRANI A G, NOVELLI V, SHEEHAN S, et al. Probing the redox states at the surface of electroactive nanoporous NiO thin films[J] . ACS Applied Materials & Interfaces,2014,6:143 − 152. [21] STANNORE B R, BRILHAC J F, GILOT P. The oxidation of soot: A review of experiments, mechanisms and models[J] . Carbon,2001,39:2247 − 2268. doi: 10.1016/S0008-6223(01)00109-9 [22] KIM H R, CHOI K I, KIM K M, et al. Ultra-fast responding and recovering C2H5OH sensors using SnO2 hollow spheres prepared and activated by Ni templates[J] . Chemical Communications,2010,46:5061 − 5063. doi: 10.1039/c0cc00213e [23] WU E H, FENG X S, ZHENG Y B, et al. Inverse coprecipitation directed porous core-shell Mn-Co-O catalyst for efficient low temperature propane oxidation[J] . ACS Sustainable Chemistry & Engineering,2020,8:5787 − 5798. -






 下载:
下载: