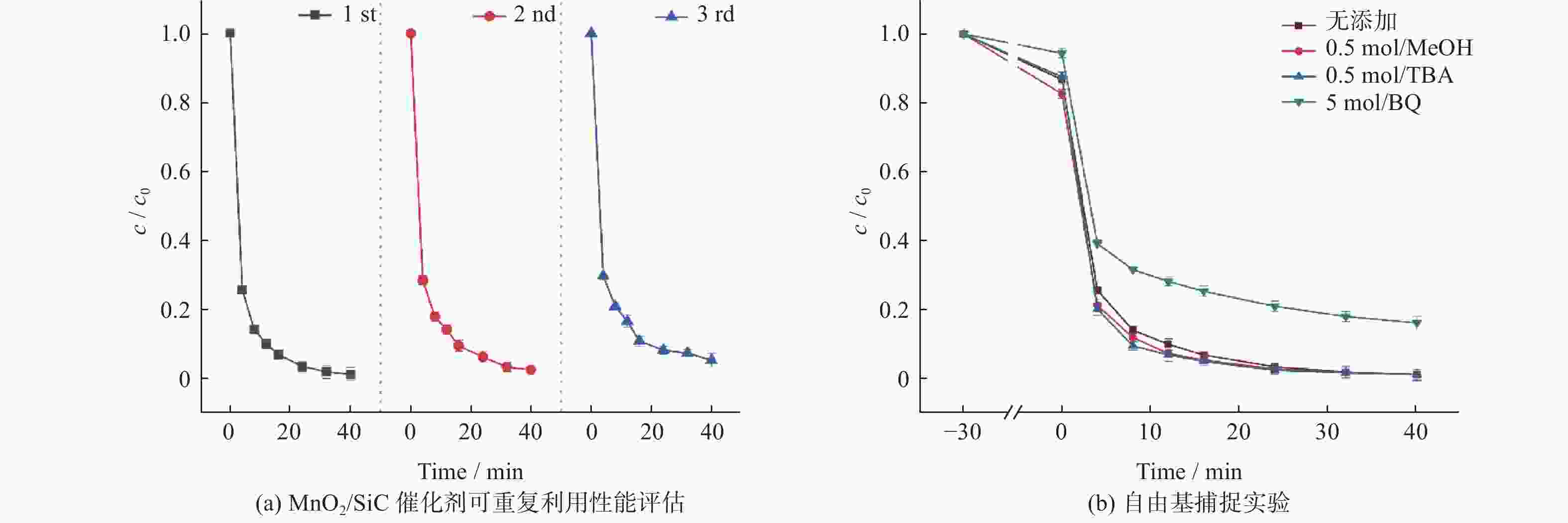
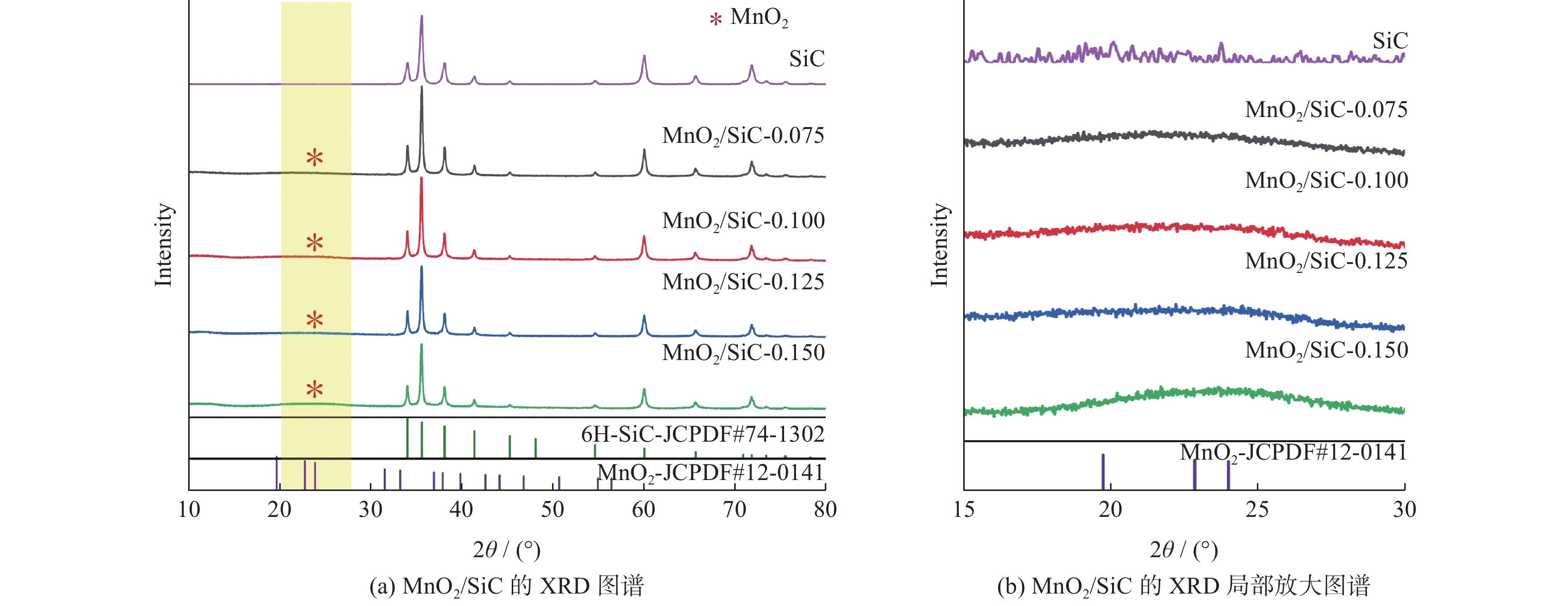
|
[1]
|
蒋婧, 程锦程. 多孔柱[5]芳烃聚合物的制备及其对有机污染物的吸附性能研究[J] . 铜陵学院学报,2018,17(6):113 − 117.
|
|
[2]
|
张卡卡. 苯胺−磺酸基苯胺共聚物的制备及其对染料吸附性能的研究 [D]. 郑州: 郑州大学, 2014.
|
|
[3]
|
WU N, WANG S, LI L, et al. Degradation of organosulfur compound in wastewater of diesel refining by alkali heat activated persulfate[J] . Environmental Pollution & Control,2019,41(4):435 − 438, 444.
|
|
[4]
|
WANG Q, SHAO Y, GAO N, et al. Degradation kinetics and mechanism of 2, 4-Di-tert-butylphenol with UV/persulfate[J] . Chemical Engineering Journal,2016,304:201 − 208. doi: 10.1016/j.cej.2016.06.092
|
|
[5]
|
DAN J, WANG Q, MU K, et al. Degradation of sulfachloropyridazine by UV-C/persulfate: kinetics, key factors, degradation pathway[J] . Environmental Science: Water Research & Technology,2020,6(9):2510 − 2520.
|
|
[6]
|
CAI A, DENG J, ZHU T, et al. Enhanced oxidation of carbamazepine by UV-LED/persulfate and UV-LED/H2O2 processes in the presence of trace copper ions[J] . Chemical Engineering Journal,2021,404:127119. doi: 10.1016/j.cej.2020.127119
|
|
[7]
|
RAO Y F, QU L, YANG H S, et al. Degradation of carbamazepine by Fe(Ⅱ)-activated persulfate process[J] . Journal of Hazardous Materials,2014,268:23 − 32. doi: 10.1016/j.jhazmat.2014.01.010
|
|
[8]
|
ZHU J P, LIN Y L, ZHANG T Y, et al. Modelling of iohexol degradation in a Fe(Ⅱ)-activated persulfate system[J] . Chemical Engineering Journal,2019,367:86 − 93. doi: 10.1016/j.cej.2019.02.120
|
|
[9]
|
ANIPSITAKIS G P, DIONYSIOU D D. Degradation of organic contaminants in water with sulfate radicals generated by the conjunction of peroxymonosulfate with cobalt[J] . Environmental Science & Technology,2003,37(20):4790 − 4797.
|
|
[10]
|
LI Z Y, WANG L, LIU Y L, et al. Unraveling the interaction of hydroxylamine and Fe(Ⅲ) in Fe(Ⅱ)/Persulfate system: A kinetic and simulating study[J] . Water Research,2020,168:115093. doi: 10.1016/j.watres.2019.115093
|
|
[11]
|
JIANG C, GARG S, WAITE T D. Hydroquinone-mediated redox cycling of iron and concomitant oxidation of hydroquinone in oxic waters under acidic conditions: Comparison with iron-natural organic matter interactions[J] . Environmental Science & Technology,2015,49(24):14076 − 14084.
|
|
[12]
|
LI T, ZHAO Z, WANG Q, et al. Strongly enhanced Fenton degradation of organic pollutants by cysteine: An aliphatic amino acid accelerator outweighs hydroquinone analogues[J] . Water Research,2016,105:479 − 486. doi: 10.1016/j.watres.2016.09.019
|
|
[13]
|
王一凡, 李小蝶, 侯美茹, 等. 锰基氧化物活化过硫酸盐降解水中有机污染物的研究进展[J] . 环境科学研究,2021,34(8):1899 − 1908. doi: 10.13198/j.issn.1001-6929.2021.04.02
|
|
[14]
|
MORKOÇ H, STRITE S, GAO G B, et al. Large-band-gap SiC, III–V nitride, and II–VI ZnSe-based semiconductor[J] . Journal of Applied Physics,1994,76(3):1363 − 1398. doi: 10.1063/1.358463
|
|
[15]
|
Goldberg Y, LEVINSHTEIN M E, RUMYANTSEV S L. Properties of advanced semiconductor materials: GaN, AlN, SiC, BN, SiC, SiGe [M]. Lexington: ScienceOpen, 2001: 93–148.
|
|
[16]
|
PRESSER V, NICKEL K G. Silica on silicon carbide[J] . Critical Reviews in Solid State and Materials Sciences,2008,33(1):1 − 99. doi: 10.1080/10408430701718914
|
|
[17]
|
OHTANI N, TAKAHASHI J, KATSUNO M, et al. Development of large single-crystal SiC substrates[J] . Electronics and Communications in Japan,1998,81(6):8 − 19.
|
|
[18]
|
ZIEGLER G, LANIG P, THEIS D, WEYRICH C. Single crystal growth of SiC substrate material for blue light emitting diodes[J] . IEEE Transactions on Electron Devices,1983,30(4):277 − 281. doi: 10.1109/T-ED.1983.21117
|
|
[19]
|
FALK A L, BUCKLEY B B, CALUSINE G, et al. Polytype control of spin qubits in silicon carbide[J] . Nature Communication,2013,4:1819. doi: 10.1038/ncomms2854
|
|
[20]
|
PEDERSEN H, LEONE S, KORDINA O, et al. Chloride-based CVD growth of silicon carbide for electronic applications[J] . Chemical Reviews,2012,112(4):2434 − 2453. doi: 10.1021/cr200257z
|
|
[21]
|
杨俊晖, 张惠灵, 梁俊杰, 等. 壳聚糖/磁性榴莲生物炭对亚甲基蓝的吸附研究[J] . 环境科学与技术,2021,44(12):7 − 12. doi: 10.19672/j.cnki.1003-6504.1877.21.338
|
|
[22]
|
KHAN A, ZHANG K K, SUN P, et al. High performance of the α-Mn2O3 nanocatalyst for persulfate activation: Degradation process of organic contaminants via singlet oxygen[J] . Journal of Colloid and Interface Science,2021,584:885 − 899. doi: 10.1016/j.jcis.2020.10.021
|
|
[23]
|
ZHOU X, LUO H P, SHENG B, et al. Cu2+/Cu+ cycle promoted PMS decomposition with the assistance of Mo for the degradation of organic pollutant[J] . Journal of Hazardous Materials,2021,411:125050. doi: 10.1016/j.jhazmat.2021.125050
|
|
[24]
|
GOVINDAN K, RAJA M, NOEL M, et al. Degradation of pentachlorophenol by hydroxyl radicals and sulfate radicals using electrochemical activation of peroxomonosulfate, peroxodisulfate and hydrogen peroxide[J] . Journal of Hazardous Materials,2014,272:42 − 51. doi: 10.1016/j.jhazmat.2014.02.036
|







 下载:
下载: